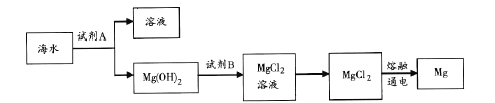
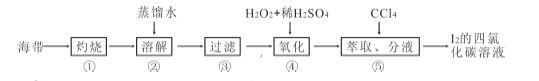
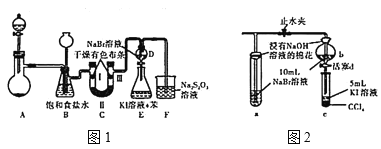
��Ŀ����
����Ŀ������������(NOSO4H)��Ҫ����Ⱦ�ϡ�ҽҩ�ȹ�ҵ��ʵ��������ͼװ��(�г�װ����)��ȡ������NOSO4H���������Ʒ���ȡ���֪��NOSO4H��ˮˮ�⣬������Ũ��������ֽ⡣

��1������װ��A��ȡSO2�����������˵��Լ���_____(��������ĸ���)
A��Na2SO3�����20%���� B��Na2SO3�����20%����
C��Na2SO3�����70%���� D��Na2SO3�����18.4mol/L����
��2��װ��B��ŨHNO3��SO2��ŨH2SO4�����·�Ӧ�Ƶ�NOSO4H��
��Ϊ�˿���ͨ��SO2�����ʣ����Բ�ȡ�Ĵ�ʩ��_______��
�ڸ÷�Ӧ����ά����ϵ�¶Ȳ�����20�档���¶ȹ��ߣ����ʽ��͵Ŀ���ԭ����____��
�ۿ�ʼ��Ӧ����������������NOSO4H���¶ȱ仯������Ӧ�������Լӿ죬��ԭ�������______��
��3����ʵ��װ�ô��ڿ��ܵ���NOSO4H�������͵�ȱ����______��
��4���ⶨNOSO4H�Ĵ���
ȷ��ȡ1.337 g��Ʒ����250 mL����ƿ�У�����0.1000mol/L��60.00 mL��KMnO4����Һ��10 mL 25%H2SO4��Һ��Ȼ��ҡ�ȡ���0.2500 mol/L�����Ʊ���Һ�ζ������IJ�������Һ�����Ϊ20.00 mL��
��֪��2KMnO4��5NOSO4H��2H2O��K2SO4��2MnSO4��5HNO3��2H2SO4
����ƽ��__MnO4-��___C2O42-��______��___Mn2+��____��__H2O
������������Ĵ���=___%(������������λ��Ч����)��
���𰸡�C ���ڷ�Һ©������������Ũ����ĵμ��ٶ� ŨHNO3�ֽ⡢�ӷ� ���ɵ�NOSO4H��Ϊ�÷�Ӧ�Ĵ��� C(��A)�е�ˮ���������B��ʹ��Ʒ����ˮ�� 2 5 16H+ 2 10CO2�� 8 95
��������
���������֪����װ��B������ŨHNO3��SO2��ŨH2SO4�����·�Ӧ�Ƶ�NOSO4H������װ��A��ȡ������������װ��C���ն���Ķ�������ֹ��Ⱦ�������ݴ˷�����
��1����װ�����ڹ�Һ��ҺҺ��ϲ�������װ�á�Na2SO3��Һ��HNO3��Ӧʱ������������������Ʊ�������ԭΪһ��������ѡ��A�����ϣ�ʵ�����ƶ��������ԭ���ǻ���SO32-��H+�ķ�Ӧ����Ϊ���ɵ�SO2������ˮ�����Բ�����ϡ��������ȡ������������SO2���ݳ������������Ũ���ᣨ18.4mol/L�����������ǹ��ۻ����Ũ��Խ�ߣ�Խ��������������H+�����Բ��ʺ���̫Ũ����������ȡSO2��ѡ��B��ѡ��D�����ϣ�ѡ��C���ϣ���ѡC��
��2��װ��B��ŨHNO3��SO2��ŨH2SO4�����·�Ӧ�Ƶ�NOSO4H��
��Ϊ�˿���ͨ��SO2�����ʣ����Բ�ȡ�Ĵ�ʩ�ǵ��ڷ�Һ©������������Ũ����ĵμ��ٶȣ�
�ڸ÷�Ӧ����ά����ϵ�¶Ȳ�����20�档���¶ȹ��ߣ�ŨHNO3�ֽ⡢�ӷ������ʽ��ͣ�
�ۿ�ʼ��Ӧ����������������NOSO4H���¶ȱ仯�������ɵ�NOSO4H��Ϊ�÷�Ӧ�Ĵ�����ʹ��Ӧ�������Լӿ죻
��3����ʵ��װ�ô��ڿ��ܵ���NOSO4H�������͵�ȱ����C(��A)�е�ˮ���������B��ʹ��Ʒ����ˮ�⣻
(5)�ٷ�������MnO4����C2O42����������ԭ��Ӧ��MnO4����������������ԭ������Mn2+��C2O42������ԭ�����������ɳǶ�����̼����ϵ�ʧ�����غ�͵���غ�ɵõ�MnO4����C2O42�������ӷ�Ӧ����ʽΪ��2MnO4��+5C2O42��+16H+��2Mn2++10CO2��+8H2O��
�ڸ��������֪������KMnO4��Һ����NOSO4H��Ӧ�����ò�������Һ�ζ�ʣ������KMnO4��Һ����0.2500mol��L��1�����Ʊ���Һ�ζ�����KMnO4��Һ�����IJ�������Һ�����Ϊ20.00mL����֪ʣ���KMnO4�����ʵ���n1(MnO4��)=![]() n(C2O42��)=
n(C2O42��)=![]() ��0.2500mol��L��1��20.00��10-3L=2��10-3mol�����������������ĵ�KMnO4�����ʵ���n2(MnO4��)=0.1000mol��L��1��60.00��10-3L-2��10-3mol=4��10-3mol��n(NOSO4H)=5/2n2(MnO4��)=10-2mol������������Ĵ���=m(NOSO4H)/1.337g��100%=10-2mol��127 g��mol��1/1.337g��100%=95.0%��
��0.2500mol��L��1��20.00��10-3L=2��10-3mol�����������������ĵ�KMnO4�����ʵ���n2(MnO4��)=0.1000mol��L��1��60.00��10-3L-2��10-3mol=4��10-3mol��n(NOSO4H)=5/2n2(MnO4��)=10-2mol������������Ĵ���=m(NOSO4H)/1.337g��100%=10-2mol��127 g��mol��1/1.337g��100%=95.0%��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�����Ŀ������ʵ������ܴﵽ��Ӧʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ̽��I-��Fe2+�Ļ�ԭ��ǿ�� | ��FeCl3ϡ��Һ�����μ���KI��Һ������ |
B | ̽��HPO42-��ˮ��Һ�еĵ���̶���ˮ��̶ȵ���Դ�С | �ⶨ0.1 mol��L-1NaH2PO4��Һ��pH |
C | ����0.1 mol��L-1�ڱ����������( | ��ȡ5.1 g�ڱ�������������ձ���,������������ˮ�ܽ�,ת����500 mL����ƿ�ж��� |
D | �Ƚ�CaCO3��CaSO4��Ksp��С | �����ʯ��ˮ�е���0.1 mol��L-1Na2CO3��Һ�������г�������,�ٵμ�0.1 mol��L-1Na2SO4��Һ |
A.AB.BC.CD.D