��Ŀ����
����Ŀ��Ϊ̽��ij���� X �Ը����ڳ�ʪ�����µĸ�ʴ��Ӱ�����ã�ijͬѧ����������ʵ�飺
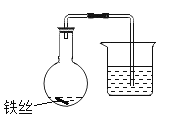
�� ѡ�� 6 ��������ͬ����ƿ���ֱ���Ϊ 1~6��������������ͬ����˿��
�� �� 1~3 ��ƿ�з��� 2mL ��ˮ���� 4~6 ��ƿ�з��� 2mL10%X ��ˮ��Һ������˿��ʪ��
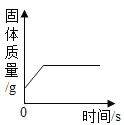
�� ���ϴ��е��ܵ����ӣ�������ĩ�˽���ʢ��ˮ���ձ��У���¼������Һ��ij�ʼλ�ã���ͼ��ʾ����
�� �������»����£�ÿ��һСʱ����һ�Σ�����Ϊ 3 Сʱ����¼���ݡ�������±���ʾ��
ʱ�䣨Сʱ�� | 1 ��ƿ | 2 ��ƿ | 3 ��ƿ | 4 ��ƿ | 5 ��ƿ | 6 ��ƿ |
0 | 0 | 0 | 0 | 0 | 0 | 0 |
1 | 0 | 0.1 | 0 | 1.2 | 1.4 | 0 |
2 | 0.8 | 0.7 | 0.9 | 5.6 | 5.7 | 0.1 |
3 | 3.5 | 3.2 | 3.7 | 9.8 | 10.0 | 0.1 |
ע�����ù����е�����Һ�������������λ��cm��
��1���ڸ���̽���У�Ҫȡͬ�����õ� 3 ֻ��ƿ��Ŀ����_��
��2��6 ��ƿ������������ 4��5 ��ƿ��ͬ������ԭ����_��
��3��ͨ����ʵ�飬�ɳ����ó��Ľ�����_��
��4������̽��������Ƿ���ڲ���_����θĽ�_��
���𰸡�������ʹ���۸��ɿ� װ��©������װ�������Բ �ڳ�ʪ���������� X ����ٸ����ĸ�ʴ�� ���ڲ��� ����3����ͬ��3����ƿ�о�����2mL10%X ��ˮ��Һ����Ϊ�Ա�ʵ��
��������
��1���ڸ���̽���У�����һֻ��ƿ�����ۿ��ܾ���żȻ�ԣ�ȡͬ�����õ�3ֻ��ƿ���Լ�����ʹ���۸��ɿ���
��2��6 ��ƿ������������ 4��5 ��ƿ��ͬ��������Һ��û������������ԭ����װ��©������װ�������Բ��
��3��ͨ����ʵ�飬�ɳ����ó��Ľ����ǣ��ڳ�ʪ���������� X ����ٸ����ĸ�ʴ��
��4������̽������ƴ��ڲ��㣬�Ľ�Ϊ������3����ͬ��3����ƿ�о�����2mL10%X ��ˮ��Һ����Ϊ�Ա�ʵ�顣

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�����Ŀ���±��Ǽ���������20�����ܽ�Ⱥ���Է����������ش��������⣺
���� | NaCl | NaHCO3 | NH4Cl | NH4HCO3 |
�ܽ��/g | 36.0 | 9.6 | 37.2 | 21.0 |
��Է������� | 58.5 | 84 | 53.5 | 79 |
��1��20��ʱ����50g����ˮ���Ʊ���NaHCO3��Һ��
�ټ��������NaHCO3��������_____g��
�ڽ�������NaHCO3���嵹��ʢ��50g����ˮ���ձ��У�Ȼ����_____�����������ƣ����Ͻ��裬ֱ��_____��
�����������õ���Һ�ɴ������ʵ���������Ϊ1%��ϡH2SO4_____g��
��2����ͬ�¶��£���ͬ����ͬʱ�ܽ���ͬһ�ܼ��У��ܽ�Ȳ��䡣��20��ʱ����11.7gNaCl�����15.8gNH4HCO3����ͬʱ����ʢ��100gˮ���ձ��У���ֽ��裬���ã��۲쵽�ձ��ײ��о�����֣��ù���ֻ�������ֽⷴӦ�����ľ�����_____���������������_____g��