��Ŀ����
����Ŀ����ʯ����CaO��NaOH�Ĺ������ͨ����������CO2���ɲ����塣ijѧУʵ��С����ʵ����ȡ��һ�����Ѿ�ʹ�ù��ļ�ʯ����Ʒ��������ɷֽ���������̽����
[�������]�ü�ʯ����Ʒ�п��ܺ���CaO��___________ (�ѧʽ)��CaCO3��NaOH��Na2CO3.
[��Ʒ���] ��1����ͬѧ���ջ��з��������ĸü�ʯ����Ʒ���ȼ�������ˮ��δ���ַ��������ټ�����������ˮ��ֽ��裬���ã��а�ɫ�������֣���ͬѧ��Ϊ��Ʒ��һ������CaCO3.����Ϊ��ͬѧ�Ľ����Ƿ��Ͻ�?������:______________________________��
��2����ͬѧ��һ�����ʵ�鲢������֤���������±�:
ʵ����� | ʵ������ | ʵ����� |
�� �Ӽ�ͬѧ���ձ���ȡ�����ϲ���Һ ���Թ��У������еμ�����________��Һ�� | �а�ɫ�������� | ��Ʒ��һ����Na2CO3 |
�� �����������õ��Ļ������ˣ�����Һ�еμ���ɫ��̪��Һ�� | ���������� | ��Һ�в�����NaOH |
[ʵ�����]����Ϊͨ�����ϼס�����λͬѧ��ʵ��̽�����Ƿ����ȷ���ü�ʯ����Ʒ����ɳɷ�?���ܣ���д������ɣ������ܣ���˵������______________________________��
[ʵ�鷴˼]��ͬѧ��һ���������ͼ��ʾ��ʵ��װ�ã�ͨ������Bװ�õ������仯���ⶨһ������ Ʒ������ϡ���ᷴӦ�����ɵ�CO2������(���������ã�ÿ������ȫ��Ӧ����������)������ͬѧ���ղ�õ�CO2������ʵ��ֵС������Ϊ����ԭ����____________________��
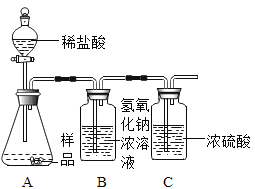
���𰸡�Ca��OH��2 �������������ƺ�̼���Ʒ�Ӧ�������ɵ�̼��� CaCl2 �ܣ�CaCO3��Na2CO3 ��ƿ�в�������CO2δ��NaOH��Һ���գ�����ƿ��ˮ�����˲���CO2��
��������
[�������]����������ˮ��Ӧ�����������ƣ����Կ��ܻ������������ƣ���ѧʽΪ��Ca��OH��2��
[��Ʒ���] ��1����ͬѧ���ձ��з��������ļ�ʯ����Ʒ��������������ˮ��ֽ��裬�����а�ɫ��������ͬѧ��Ϊ��Ʒ��һ������CaCO3����˵����ȷ����Ϊ��ʯ����Ʒ�п��ܺ���̼���ƺ��������ƣ�̼�������������Ʒ�Ӧ������̼��Ƴ�����
��2������ͬѧҪ��֤���Ƿ���̼���ƣ���̼�������Ȼ��Ʒ�Ӧ������̼��ư�ɫ��������˿ɴӼ�ͬѧ���ձ���ȡ�����ϲ���Һ���Թ��У������еμ�����CaCl2��Һ���а�ɫ�������ɣ�˵������̼���ƣ�
[ʵ�����]��ͬѧ���ջ��з��������ĸü�ʯ����Ʒ���ȼ�������ˮ��δ���ַ�������������Ʒ��û�������ƣ������������õ��Ļ������ˣ�����Һ�еμ���ɫ��̪��Һ��������������Һ�в������������ƺ��������ƣ�������Ʒ��û���������ƺ��������ƣ��ʹ�����һ������̼��ƺ�̼���ƣ�
[ʵ�鷴˼]������ƿ�в�������CO2δ��NaOH��Һ���ջ���ƿ��ˮ�����˲���CO2�����Ա�ͬѧ���ղ�õ�CO2������ʵ��ֵС��

����Ŀ��С��ͬѧ����һƿ���ڷ����Ѿõ�NaOH��Һ������һЩʵ��̽����
��������⣩��ƿNaOH��Һ���ʳ̶�����أ�
��������룩С���IJ��룺NaOH��Һ���ֱ��ʡ�
��1������²��룺______________��
��ʵ��̽����С���������ʵ������֤�Լ��IJ��룬����ݱ���������д
��2��С��ʵ��ʱ������
ʵ�鲽�� | ���� | ���� |
ȡ����NaOH��Һ��Ʒ���Թ����ȵμ�������CaCl2��Һ��Ȼ���ٵμӷ�̪��Һ | _______��_______ �� | NaOH��Һ���ֱ��� |
��3�� ������IJ�����ȷ������С����ʵ�鷽������ʵ�飬������˿���������ɫ����֮�⣬���ܿ�����ʵ��������__________��
��4����ʵ�鷴˼���������ʢ�Ca(OH) 2��Һ����Ba(NO3)2��Һ����Ba(OH) 2��Һ�������С��ʵ����CaCl2��Һ����________ (�����)��
��5������չӦ�ã�ͨ��ʵ��ʵ�飬ȷ����ƿ�����Ѿõ�NaOH��Һ���ֱ��ʡ���ȡ100g������Һ����������μ���11.1%��CaCl2��Һ��������������������CaCl2��Һ�����Ĺ�ϵ��ͼ��ʾ��
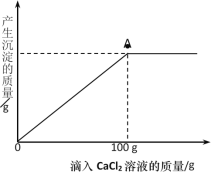
��A�㴦������Һ��������_____________________��
���������Һ��Na2CO3������������______________��д��������̣�