��Ŀ����
����Ŀ��ijС��ͬѧ��ʵ���ҽ���ʵ�飬������롣
��1��ͬѧ����ȡ�����ڿ����о��õ��������ƹ�����Ʒ��ˮ���Ƴ���Һ����ȡ2mL����Һ��С�ձ��У�Ȼ��μӼ��η�̪��Һ������ʱ�۲쵽��ʵ������Ϊ��_____________________���������ձ�����μ���ϡ���Ტ�ò��������裬��Һ����ɫ�ı䣬ͬʱ�۲쵽�����ݲ��������ۣ��������Ʊ��ʡ��뻯ѧ����ʽ�����������Ʊ��ʵ�ԭ��_____________________��
��2������������Ʊ��ʵij̶����˽�һ��̽����
���������룩����һ����Ʒ���ֱ��ʣ��ɷ�ΪNaOH��Na2CO3��
���������Ʒ��ȫ���ʣ��ɷ�Ϊ_______________��
���������ϣ���BaCl2��Һ�����ԣ�BaCO3������ˮ������̼������Һ����μ���ϡ����ķ�Ӧ�Ƿֲ���Ӧ���ᷢ������̼�����ƺ��Ȼ��Ƶķ�Ӧ��
��ʵ��̽����Ϊ��֤���룬ͬѧ�����������������������ʵ�顣
�ٶ��Է�����ƣ����㽫��ʵ����Ʋ���������
ʵ������ | ʵ������ | �ó����� |
ȡ������Ʒ��Һ����������BaCl2��Һ�����ã����ϲ���Һ�еμӼ�����ɫ��̪��Һ | ______________ | ����һ��ȷ |
�ڶ���������� ��ȷ��ȡ 11.95g ���ʵ�NaOH��Ʒ������ƿ�У��õ��ӳӳƵ���ƿ����Ʒ��������Ϊ46.95g���ٰ� 150.00 g7.3 ��ϡ����ƽ���ֳ� 6�ȷݣ�ÿ��25.00g�����ڳ��ҡ����ƿʱ��������Ʒ�У�ÿ�γ�ַ�Ӧ���õ��ӳӳƵ���ƿ����ʢ���ʵ�������ʵ�����ݼ�¼���£�
��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� | ������ |
��ƿ����ʢ����������/g | 71.95 | 96.95 | 120.85 | 144.75 | 168.65 | 193.65 |
��ʵ����ۣ����������ʵ�����ݣ�������Ʒ���������Ƶ���������Ϊ______����ȷ��0.1%��������һ��ȷ��
����˼������
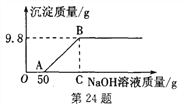
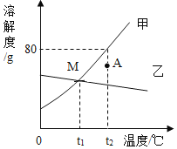
������ͼ��ֱ������ϵ�л����������������������ϡ���������Ĺ�ϵ����______��
�����жϵڶ��μ�����������з�����Ӧ�Ļ�ѧ����ʽ_________________��ԭ��______________________��
���𰸡���Һ����ɫ��Ϊ��ɫ 2NaOH+CO2=Na2CO3+H2O Na2CO3 �а�ɫ�����������ϲ���Һ����ɫ��Ϊ��ɫ 33.5% 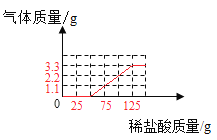 NaOH+HCl=NaCl+H2O ��Ϊʵ��������ܹ�����3.3g������̼��ͨ���������3.3g������̼������75gϡ���ᣬ��˵��������̼���Ƶķ�Ӧǡ�����ڵ�3��5�μ������ʵ������н��У������������������Ƶķ�Ӧ�����ڵ�1��2����ʵ����
NaOH+HCl=NaCl+H2O ��Ϊʵ��������ܹ�����3.3g������̼��ͨ���������3.3g������̼������75gϡ���ᣬ��˵��������̼���Ƶķ�Ӧǡ�����ڵ�3��5�μ������ʵ������н��У������������������Ƶķ�Ӧ�����ڵ�1��2����ʵ����
��������
��1����������������еĶ�����̼��Ӧ����̼���ƣ�̼���ƺ�����������Һ���Լ��ԣ�ȡ�����ڿ����о��õ��������ƹ�����Ʒ��ˮ���Ƴ���Һ����ȡ2mL����Һ��С�ձ��У�Ȼ��μӼ��η�̪��Һ������ʱ�۲쵽��ʵ������Ϊ����Һ����ɫ��Ϊ��ɫ���������ձ�����μ���ϡ���ᣬϡ�����̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���壬
�����Һ����ɫ��Ϊ��ɫ��2NaOH+CO2=Na2CO3+H2O��
[��������]����һ����Ʒ���ֱ��ʣ��ɷ�ΪNaOH��Na2CO3��
���������Ʒ��ȫ���ʣ��ɷ�ΪNa2CO3��
[ʵ��̽��]��
ʵ������ | ʵ������ | �ó����� |
ȡ������Ʒ��Һ����������BaCl2��Һ�����ã����ϲ���Һ�еμӼ�����ɫ��̪��Һ | �а�ɫ�����������ϲ���Һ����ɫ��Ϊ��ɫ | ����һ��ȷ |
���Na2CO3���а�ɫ�����������ϲ���Һ����ɫ��Ϊ��ɫ��
�ڵ���μ���ϡ����ʱ��ϡ�����̼���Ʒ�Ӧ�����ɶ�����̼������Ϊ46.95g+125g-168.65g=3.3g������Ʒ��̼���Ƶ�����Ϊx��
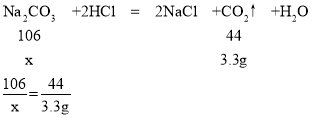
x=7.95g
��Ʒ���������Ƶ���������Ϊ![]() ��
��
���33.5%��
[��˼����]
�ٲ���������̼���������������ϡ���������Ĺ�ϵ������ͼ

��ʵ��������ܹ�����3.3g������̼��ͨ���������3.3g������̼������75gϡ���ᣬ��˵��������̼���Ƶķ�Ӧǡ�����ڵ�3��5�μ������ʵ������н��У������������������Ƶķ�Ӧ�����ڵ�1��2����ʵ���У�ϡ������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ����ѧ����ʽΪNaOH+HCl=NaCl+H2O��
��� ��
��
NaOH+HCl=NaCl+H2O��ʵ��������ܹ�����3.3g������̼��ͨ���������3.3g������̼������75gϡ���ᣬ��˵��������̼���Ƶķ�Ӧǡ�����ڵ�3��5�μ������ʵ������н��У������������������Ƶķ�Ӧ�����ڵ�1��2����ʵ���С�

 ��У����ϵ�д�
��У����ϵ�д�