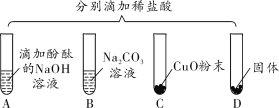
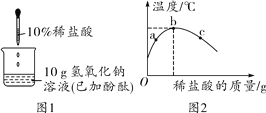
��Ŀ����
����Ŀ��������ʵ���ҳ��õ��Լ���Ҳ����Ҫ�Ļ���ԭ�ϣ�����ͬѧ���ɳ������������ѧ���ʣ���ͼ1��ʾ�����߱�ʾ���Ӧ��������ͬѧΪ��ʾ��Ӧʵ�ʻ�����ͼ2��
�����ͼʾ�ش��������⡣
��1����ͼ1��ʾ����������ɫʯ����Һ�μӵ������У���Һ��_____ɫ��
��B��������_____������ţ���
a��Mg������b��Fe������c��Ag������d��Zn
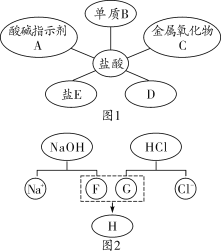
����CΪ�������Ҫ�ɷ֣�C�����ᷴӦ�Ļ�ѧ����ʽ��_____��
��D����ʾ�����������_____��
����EΪ̼���ƣ�D��E��Ӧ�Ļ�ѧ����ʽ��_____��
��2����ͼ2��ʾ����ͼ����������������Һ�����ᷴӦ����ʵ�ʡ���ͼ2�У�F��G��H��Ӧ�������Ļ�ѧ��������Ϊ_____��д���÷�Ӧ�Ļ�ѧ����ʽ_____��
��3����ϡ�����зֱ������������ʣ���ҺpH�����������仯����_____������ţ���
A AgNO3����
B Ũ����
C Ca��OH��2����
D H2O
���𰸡��� c Fe2O3��6HCl=2FeCl3��3H2O �� Ca��OH��2��Na2CO3=CaCO3����2NaOH���������ɣ� OH����H����H2O HCl��NaOH=NaCl��H2O A
��������
��1����������ɫʯ����Һ�μӵ������У������ʹ��ɫʯ����Һ��ɺ�ɫ����ֻ���ڽ������˳����������ǰ�Ľ����������ᷢ����Ӧ��������������Բ������ᷴӦ�����B���������������������Ҫ�ɷ�Ϊ�������������ᷴӦ�����Ȼ�����ˮ����Ӧ�Ļ�ѧ����ʽΪFe2O3��6HCl=2FeCl3��3H2O�����ỹ��������Ӧ������DΪ�����EΪ̼���ƣ�D��Ϊ�������ơ����������ȣ���Ӧ�Ļ�ѧ����ʽΪCa��OH��2��Na2CO3=CaCO3����2NaOH�ȡ���2���������������ᷴӦ��ʵ�������������Ӻ������ӽ������ˮ���ӣ�����F��G��H���ֱ�ΪOH����H����H2O���÷�Ӧ���������������ᷴӦ�����Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪHCl��NaOH=NaCl��H2O����3����������ϡ���ᷴӦ�����Ȼ������������ᣬ��������Ŀû�иı䣬��ҺpH�������䣬A��ȷ������Ũ�����ʹ������ǿ����ҺpH��С��B�����������ƻ��к�ϡ�����е������ӣ�ʹ��ҺpH���C����ˮ��ϡ��ϡ���ᣬʹ��Һ��pH���D����

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�����Ŀ������ʵ������ܴﵽʵ��Ŀ�ĵ��ǣ�������
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ��ȥ����Ʒ��������� | ������Ʒ��ʱ�������ϡ������ |
B | ����ij��Һ���Ƿ��� | �μ��Ȼ�����Һ |
C | �������ᡢ�Ȼ��ء��Ȼ���������Һ | �μ�̼������Һ |
D | �����Ȼ��ƺ��Ȼ�þ���� | �ܽ⣬������������������Һ�����ˣ������ᾧ |
A.AB.BC.CD.D
����Ŀ��(2017ʯ��ׯ�ʼ��ģ)ij�о���ѧϰС�����ⶨ�����еĺ�̼�������õķ������£�ȡ������Ʒ5.8 g����50 gϡ�����5�μ��뵽������Ʒ�У�ʵ������е����ݼ��±�(����������Ʒ��ֻ������̼)������㣺
��������Ĵ��� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
ϡ��������/g | 10 | 10 | 10 | 10 | 10 |
ʣ���������/g | 4.4 | 3.0 | 1.6 | 0.2 | 0.2 |
��1��5.8 g�����к�̼������Ϊ________g��
��2������ϡ���������ʵ�����������
����Ŀ����2018���ڸıࣩijCa��OH��2��Ʒ�ѱ��ʣ���ֻ��CaCO3һ�����ʣ�ȡ10 g����Ʒ������Ʒ���뵽�����������У������������±���
ʱ��/s | t0 | t1 | t2 | t3 |
�ձ���ʣ�����ʵ�����/g | 280 | 278.9 | 277.8 | 277.8 |
��1�����ɶ�����̼������Ϊ_____g��
��2������Ʒ��Ca��OH��2������������
����Ŀ���������ʵ��ܽ�����±���ʾ�����ݱ�����Ϣ�ش��������⣺
�¶�/�� �ܽ��/g | 0 | 20 | 40 | 60 | 80 | 100 |
Li2CO3 | 1.54 | 1.33 | 1.17 | 1.01 | 0.85 | 0.72 |
NaCl | 35.7 | 36 | 36.6 | 37.3 | 38.4 | 39.8 |
KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 | 245 |
��1���ܽ�����¶����߶���С����_______ �����ѧʽ��
��2������ع����л��������Ȼ��ƹ�����������û�����ø���������أ� ���ڽϸ��¶�ʱ�Ƴɱ���Һ��Ȼ��_______��
��3������ 20��ʱ�Ȼ��Ƶı�����Һ 68g����ˮ_______ g��