��Ŀ����
����Ŀ��ѧϰС��ⶨij��ҵ��ˮ(����H2SO4��HNO3������������)��H2SO4�ĺ�����ȡ100g��ˮ���ձ��У�����100g BaCl2��Һ��ǡ����ȫ��Ӧ�������˵õ�176.7g��Һ��
��1����ַ�Ӧ�����ɳ���������Ϊ______g��
��2���ù�ҵ��ˮ���������������Ϊ����?(д���������)______��
��3��Ϊ���ҵ��ˮ��Ⱦ�������ŷ�ǰӦ�Է�ˮ�����кʹ�����������������_______��
���𰸡���1��23.3����2��9.8%����3����������
��������
��ҵ��ˮ(����H2SO4��HNO3������������)��H2SO4��BaCl2��Ӧ�������ᱵ��������������ᱵ����������Ӧ��
��1����ַ�Ӧ�����ɳ���Ϊ���ᱵ��������Ϊ100g +100g -176.7g=23.3g��
��2���⣺�蹤ҵ��ˮ�����������Ϊ![]() ��
��
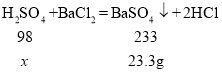
![]() =
=![]()
��ã�![]() =9.8g
=9.8g
������ϡ���������ʵ���������Ϊ![]()
![]() 100%=9.8%
100%=9.8%
������ϡ���������ʵ���������Ϊ9.8%��
��3���ù�ҵ��ˮ���д������ᣬΪ���ҵ��ˮ��Ⱦ�������ŷ�ǰӦ�Է�ˮ�����кʹ������������������������ƣ��������ƺ�ϡ���ᷴӦ��������ƺ�ˮ�������ᷴӦ��������ƺ�ˮ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ