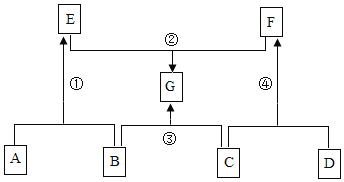
��Ŀ����
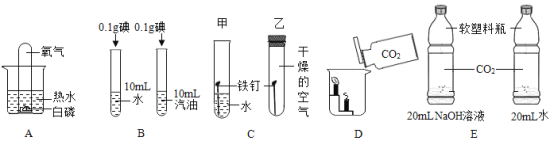
����Ŀ����ˮƿ�þú�ƿ���ڱڸ���һ��ˮ������ɷ�Ϊ̼��ơ�������þ������ˮ�� ʵ��������һƿˮ����Ʒ��Ϊ�ⶨ���и��ɷֵ�����������ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨��װ�����������ã�A��C��D��E ����װҩƷ����������֪��ʯ���������ƺ��������ƵĻ������������£�������þ�ֽ⣬��Ӧ����ʽΪ��Mg��OH��2![]() MgO + H2 O��
MgO + H2 O��
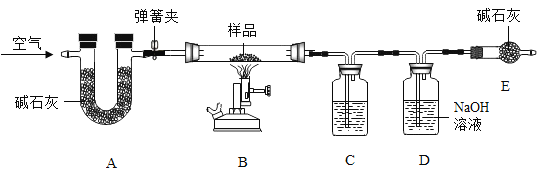
����ʵ�鲽�����£�
��.������Ϊ m ����Ʒװ��װ�� B �IJ������У���ͼ���Ӻ�װ�ã��رյ��ɼУ�����Ʒ���ȣ�
��.����Ʒ��ȫ��Ӧ���ȴ��ɼ�ͨ���������Ϩ��ƾ��ƣ�ֱ����������ȴ��
��.ʵ����ϣ����װ�� C��D �е�Һ�������ֱ�������m1��m2��
��.����ʵ���������ݼ������Ʒ��̼��ơ�������þ��ˮ������������ ��ش��������⣺
��1��װ�� C ����װҩƷΪ____________��
��2��װ�� E ������Ϊ____________��
��3��װ�� D �з�Ӧ�ķ���ʽΪ____________��
��4����ˮ����Ʒ��̼��Ƶ�������������ʽΪ____________��
��5��ʵ�鷴˼��ʵ�鲽�費�䣬��û��װ�� A���ᵼ��̼��ƵIJⶨ���____________������ƫ��������ƫС���������жϣ���ͬ��������û��װ�� E���ᵼ��������þ�IJ�ý��____________��
���𰸡�Ũ���� ���տ����еĶ�����̼��ˮ������ֹ����װ��D�� CO2 + 2NaOH = Na2CO3 + H2O ![]() ƫ�� ƫС
ƫ�� ƫС
��������
װ��A�еļ�ʯ����Ҫ��Ϊ���ų������е�ˮ�����Ͷ�����̼�ĸ��ţ���װ��B�м�����Ʒ��������������̼��ˮ������ʵ��Ŀ����Ϊ�˼������Ʒ��̼��ơ�������þ��ˮ����������������������Ҫ������ɵ�ˮ�Ͷ�����̼��������
��1��װ��D�е�����������Һ��Ҫ��Ϊ�˲�����ɵĶ�����̼������������װ��C�е���ҺӦ�ÿ��Բ�����ɵ�ˮ����������ӦΪŨ���ᣬ���Ũ���ᡣ
��2��װ��E�����տ����еĶ�����̼��ˮ������ֹ����װ��D�Բ���������Ӱ�죬������տ����еĶ�����̼��ˮ������ֹ����װ��D��
��3��װ��D�е�����������Һ�������ɵĶ�����̼����̼���ƺ�ˮ���仯ѧ����ʽΪCO2 + 2NaOH = Na2CO3 + H2O�����CO2 + 2NaOH = Na2CO3 + H2O��
��4��DҺ�����ӵ�����ʵ������̼��Ʒֽ����ɵĶ�����̼����������̼�������Ϊx��
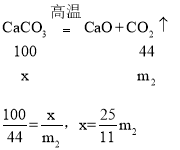
��ˮ����Ʒ��̼��Ƶ�������������ʽΪ��![]()
��5����û��װ�� A�������еĶ�����̼�ᱻD���գ�m2ƫ��̼�������ƫ�ᵼ��̼��ƵIJⶨ���ƫ����û��װ�� E�������е�ˮ����������̼����D��ͬ���ᵼ��m2ƫ��̼�������ƫ�ᵼ��̼��ƵIJⶨ���ƫ����������þ�IJ�ý��ƫС�����ƫ��ƫС��

 ��У����ϵ�д�
��У����ϵ�д�