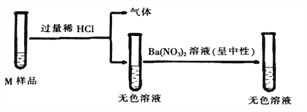
ЬтФПФкШн
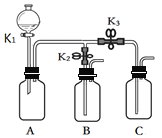
ЁОЬтФПЁПЛЏбЇаЁзщЕФЭЌбЇгУЯТЭМЫљЪОзАжУНјааШчЯТЪЕбщЃЌЪЕбщЧАK1ЁЂK2ЁЂK3ОљЮЊЙиБезДЬЌЁЃ
ФкШн ВНжш | ЪЕбщВНжш |
ЪЕбщ1 | BЁЂCжаЗжБ№ЪЂгаЮоЩЋЗгЬЊЪдвКЃЌДђПЊK1ЃЌДгЗжвКТЉЖЗжаМгШыХЈАБЫЎЃЌЙиБеK1ЃЌШЛКѓДђПЊK2ЁЂK3ЁЃ |
ЪЕбщ2 | AжаЪЂТњCO2ЃЌBжаЪЂгаЧтбѕЛЏИЦШмвКЃЌCжаЪЂгаЯЁбЮЫсЁЃ ЂйДђПЊK1ЃЌДгЗжвКТЉЖЗТ§Т§ЗХШыЪЪСПЕФЧтбѕЛЏФЦШмвК,ЙиБеK1ЁЃ ЂквЛЖЮЪБМфКѓДђПЊK2ЃЌЙлВьЕНгаAжагаГСЕэВњЩњЪБЃЌбИЫйЙиБеK2ЁЃ ЂлдйДђПЊK3ЃЌЙлВьЕНAжагаЦјХнВњЩњЁЃ |
ЃЈ1ЃЉЪЕбщ1жаЃЌПЩвдЙлВьЕНЕФЯжЯѓЪЧ__________ЁЃ
ЃЈ2ЃЉЪЕбщ2жаЃЌВНжшЂйжаЫљЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ____________________ЁЃ
ЃЈ3ЃЉЪЕбщ2жаЃЌЧыНтЪЭВНжшЂлВњЩњЦјХнЕФдвђЮЊ____________________ЁЃ
ЁОД№АИЁП BЁЂCжаЕФЮоЩЋЗгЬЊвРДЮБфКь 2NaOH+CO2 == Na2CO3+H2O AжабЙЧПМѕаЁЃЌCжаЯЁбЮЫсбиЕМЙмНјШыAжаЃЌгыЬМЫсбЮЗЂЩњЗДгІЁЃ
ЁОНтЮіЁПБОЬтПМВщСЫЖўбѕЛЏЬМЁЂЫсМюбЮЕФаджЪМАЛЏбЇЗНГЬЪНЕФЪщаДЃЌРэНтгЩгкЛЏбЇЗДгІЖјв§Ц№зАжУФкбЙЧПЕФИФБфЪЧНтЬтЕФЙиМќЁЃ
ЃЈ1ЃЉЪЕбщ1ЃКХЈАБЫЎОпгаЛгЗЂадЃЌЛсЗЂГіАБЦјЃЌЗжБ№НјШыBCСНЦПЃЌШмгкЫЎаЮГЩАБЫЎЃЌАБЫЎЯдМюадЃЌЪЙЮоЩЋЗгЬЊвРДЮБфКьЃЛ
ЃЈ2ЃЉЪЕбщ2жаЃЌВНжшЂйДгЗжвКТЉЖЗЗХШыЪЪСПЕФЧтбѕЛЏФЦШмвКЃЌЧтбѕЛЏФЦгыЖўбѕЛЏЬМЗДгІЩњГЩЬМЫсФЦКЭЫЎЃЌЛЏбЇЗНГЬЪНЮЊ2NaOH+CO2 == Na2CO3+H2OЃЛ
ЃЈ3ЃЉЪЕбщ2жаЃЌВНжшЂквЛЖЮЪБМфКѓгЩгкAжабЙЧПМѕаЁЃЌДђПЊK2ЃЌдйбИЫйЙиБеK2ЃЌЧтбѕЛЏИЦШмвКЕЙЮќШыAЦПжаЧтбѕЛЏИЦгыЬМЫсФЦЗДгІЩњГЩЬМЫсИЦГСЕэКЭЧтбѕЛЏФЦЃЛВНжшЂлОЙ§ВНжшЂкКѓAжавЛЖЈгаЬМЫсИЦГСЕэКЭЧтбѕЛЏФЦЃЛдйДђПЊK3ЃЌбЮЫсНјШыКѓгыЬМЫсИЦКЭЧтбѕЛЏФЦЖМЗДгІЁЃЬМЫсИЦгыбЮЫсЗДгІВњЩњЖўбѕЛЏЬМЁЃВНжшЂлВњЩњЦјХнЕФдвђЪЧAжабЙЧПМѕаЁЃЌCжаЯЁбЮЫсбиЕМЙмНјШыAжаЃЌгыЬМЫсбЮЗЂЩњЗДгІЁЃ

 ПЮЪБбЕСЗНЫеШЫУёГіАцЩчЯЕСаД№АИ
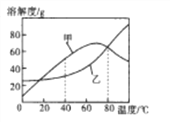
ПЮЪБбЕСЗНЫеШЫУёГіАцЩчЯЕСаД№АИЁОЬтФПЁПЯТСаЫФИіЭМЯѓжаЃЌФме§ШЗЗДгГЖдгІБфЛЏЙиЯЕЕФЪЧ
|
|
|
|
AЃЎЯђвЛЖЈСПЕФЧтбѕЛЏФЦШмвКжаЕЮМгЯЁбЮЫс | BЃЎЯђвЛЖЈСПЕФЯЁбЮЫсжаМгШыДѓРэЪЏ | CЃЎвЛЖЈСПЕФЯЁбЮЫсжаМгШыЬњЗл | DЃЎМгШШвЛЖЈСПЕФИпУЬЫсМиЙЬЬх |
A. A B. B C. C D. D