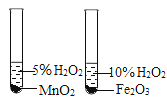��Ŀ����
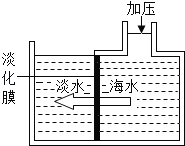
����Ŀ����Ӱ���Һ��ҵ��������������һ��Ƭ�Σ��������ǰҹ�� ��������������⣬��˶��������������ʴ�����۶ϣ�Ϊ��֤������ ��ɹ������ú��ӷ�ʽ�����µIJ�������������������ʽ������ȱ��

��һ��������dz���������Ʒ����ҵ�ϳ����豸��¯����������Ҫԭ���� ��̿��������ʯ����Ҫ�� Fe2O3����ʯ��ʯ�������ȣ���Ҫ��Ӧ�������£�

��ش�
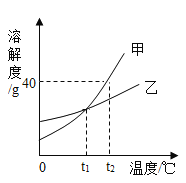
��1�������������������������ᣬ��ַ�Ӧ�����к�ɫ����ʣ�࣬�ú�ɫ����Ϊ________�� ��ѧʽ����
��2����Ӧ�ڵĻ�ѧ����ʽΪ_____���÷�Ӧ�����Ļ�����Ӧ����Ϊ_____��
��������������ʴ������
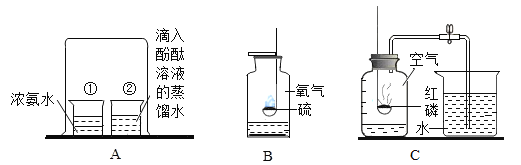
��1���������������⣬ʵ����������_______��_____________��ͬ���õĽ�����������ɫ��______���������������Ʒ�����������______������ţ���
a ��ʪ������ b ��������� c ���ֽ���ʳ��ˮ��
��2�������е�����Ʒ������Ŀ��������������������������_______�ԣ���д��һ�������з�ֹ��������ķ�����_______��
��3���������������ã�ȴ���кܺõĿ���ʴ�ԣ�ԭ����_____________ ���÷���ʽ��ʾ)
������������ɷ����ⶨ
���������ϣ�����ɷָ��ӣ���ѧʽ�ɼ�ʾΪ Fe2O3nH2O���ڼ���ʱ��ֲ���Ӧ���� ��ʧȥ�ᾧˮ���䷴Ӧ�ɱ�ʾΪ Fe2O3nH2O![]() Fe2O3+nH2O��Ũ���������ˮ����ʯ�� ��������ˮ�Ͷ�����̼��
Fe2O3+nH2O��Ũ���������ˮ����ʯ�� ��������ˮ�Ͷ�����̼��
��������⣩���⣨Fe2O3nH2O���� n ��ֵ���ڶ����أ�
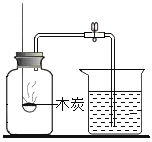
������̽����Ϊ��̽�����⣨Fe2O3nH2O������ɣ���ȡ 30.0g ���������������Ʒ����Ʒ�н������� Fe2O3nH2O������ͼ 2 ��ʾװ�ý���ʵ�顣
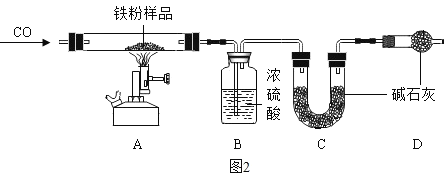
��1��Ϊ�˱�֤ʵ�鰲ȫ��ʵ�鿪ʼʱӦ��ͨ CO��Ŀ����_______��
��2����Ӧ�����������ͨ CO�����˷�ֹ���������⣬����_______ ��Ŀ�ġ�
��3��ʵ�������A �в������ڳ��ֵ�������_______ ��
��4����ָ����װ������һ�����Բ���_______ ��
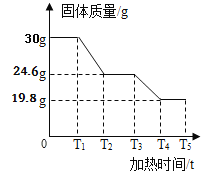
�����ݴ�����ͼ 3 �Ǽ���ʱ��� A �й���������ϵͼ���±��� B��C ���������ٱ仯ʱ B��Ũ���ᡢC �м�ʯ��װ�������仯�����
��Ӧǰ��g�� | ��Ӧ��g�� | |
B | 100 | 105.4 |
C | 150.0 | 163.2 |
��5����ȫ��Ӧ�����Ķ�����̼����Ϊ_______ �����⣨Fe2O3nH2O���� n ��ֵ��______��
��6��д�� T3-T4 ʱ����������Ļ�ѧ����ʽ_______
��7������ 30g ��Ʒ�е���������������_______����д��������̣���������� 0.1%��
���𰸡�C  ���Ϸ�Ӧ ˮ ���� ����ɫ c ������ �ྻ���� 4Al+3O2=2Al2O3 Ϊ���ž�����Ӳ���ڵĿ�������ֹCO��������Ӧ��������Σ�� ��ֹҺ�嵹�� �������ڵĹ����ɺ���ɫ��Ϊ��ɫ û��β������װ�� 13.2g 3 Fe2O3+3CO
���Ϸ�Ӧ ˮ ���� ����ɫ c ������ �ྻ���� 4Al+3O2=2Al2O3 Ϊ���ž�����Ӳ���ڵĿ�������ֹCO��������Ӧ��������Σ�� ��ֹҺ�嵹�� �������ڵĹ����ɺ���ɫ��Ϊ��ɫ û��β������װ�� 13.2g 3 Fe2O3+3CO![]() 2Fe+3CO2 28.7%
2Fe+3CO2 28.7%
��������
һ��1������������������������������̼�����������ᣬ��ַ�Ӧ��ʣ��ĺ�ɫ������̼��C��
��2����Ӧ���Ƕ�����̼�ڸ����ºͽ�̿��Ӧ����һ����̼����Ӧ�Ļ�ѧ����ʽΪ
 ��
��
�÷�Ӧ���ϡ����һ�������������ڻ��Ϸ�Ӧ��
����1���������������⣬ʵ����������ˮ��������ͬ���õĽ����
�������Ҫ�ɷ�������������ɫ�Ǻ���ɫ�ģ�
ʳ��ˮ�е�������Ũ�ȸߣ��ܹ��ƻ����Ķۻ�Ĥ���������ĸ�ʴ��������ʳ��ˮ�и�����ʴ����ѡc��
��2�������������������������ĵ����ԣ�
�����з�ֹ��������ķ����У��ྻ�����
��3���������������ã�ȴ���кܺõĿ���ʴ�ԣ�ԭ��������������Ӧ���������ܵ���������Ĥ����Ӧ�Ļ�ѧ����ʽΪ4Al+3O2=2Al2O3��
����1��ʵ�鿪ʼʱӦ��ͨ CO��Ϊ���ž�����Ӳ���ڵĿ�������ֹCO��������Ӧ��������Σ�գ�
��2����ֹҺ�嵹������������ʹ������ը�ѣ�
��3��ʵ�������A �в������ڳ��ֵ������Dz������ڵĹ����ɺ���ɫ��Ϊ��ɫ��
��4��û��β������װ�ã��ݳ���CO����Ⱦ������
��5��Cװ���������˶�����̼�Ժ�������150.0g���ӵ�163.2g������ȫ��Ӧ�����Ķ�����̼������Ϊ163.2g-150.0g=13.2g��
T1-T2ʱ������⣨Fe2O3nH2O��ʧȥ�ᾧˮ������������ʧȥ��ˮ������Ϊ30g-24.6=5.4g��һ�������������ݶ�����̼�����ݶ�����̼����Է�������Ϊ132��������̼������Ϊ13.2g��n��H2O������Ϊ5.4g��![]() �����n=3����������n ��ֵ��3��
�����n=3����������n ��ֵ��3��
��6��T3-T4 ʱ�������������һ����̼��Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ
Fe2O3+3CO![]() 2Fe+3CO2��
2Fe+3CO2��
��7����������һ����̼��Ӧ��������13.2g������̼������������£�
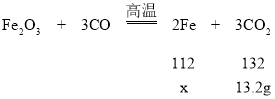
![]() �����x=11.2g����Ӧ��ɺ�����Fe������Ϊ11.2g����ͼ��֪�����Ĺ�������Ϊ19.8g������Ʒ��ԭ�е������ʵ�����Ϊ19.8g-11.2g=8.6g����Ʒ��������������Ϊ
�����x=11.2g����Ӧ��ɺ�����Fe������Ϊ11.2g����ͼ��֪�����Ĺ�������Ϊ19.8g������Ʒ��ԭ�е������ʵ�����Ϊ19.8g-11.2g=8.6g����Ʒ��������������Ϊ
![]() ��
��

����Ŀ����ͳ�ƣ��ҹ���20����90����ͷ�������Լ89���𣬸���������ش���ʧ��Ӧ�û�ѧ֪ʶ����ЧԤ���Ϳ��ƻ��֡������ͼ�����ʵ�������ԭ�����Ͳ���ȷ����( )

���ʵ�� | ���ԭ�� | |
A | סլʧ��ʱ��������Ա��ˮ��� | ���Ϳ�ȼ����Ż�� |
B | �ƾ��������Ż�ʱ����ʪĨ������ | �������������� |
C | �������Ż�ʱ���ù��Ǹ�Ϩ | �������������� |
D | ����ɭ�ֻ���ʱ�����ø���� | ��ȼ����ȼ������� |
A. A B. B C. C D. D
����Ŀ��ʵ�������ʵ�����Ӧ��ʵ��Ŀ����
|
|
|
|
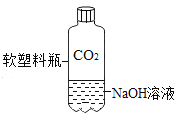
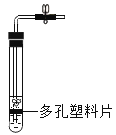
A. �ⶨ������O2 �ĺ��� | B. ֤�� CO2 ��NaOH��Һ��Ӧ | C. �Ƚ� MnO2 ��Fe2O3 �Ĵ�Ч�� | D. ��װ�þ��������շ��������Ĺ��� |
A.AB.BC.CD.D