��Ŀ����
����Ŀ��CO2ͨ��NaOH��Һ��û����������CO2��NaOH�Ƿ����˻�ѧ��Ӧ�أ�ij��ѧ��ȤС���ͬѧ�ǶԴ˽�����̽��
���������ϣ���ͨ������£�1���ˮ�ܽ�1�����CO2��
����һ��Ũ�ȵ���Һ�У����������ɵĸ��ֽⷴӦҲ�ܷ�����
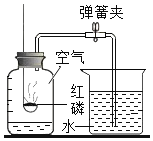
��ʵ��̽����С��ͬѧ�������ͼ��ʾ��ʵ��װ�ã���ƿ�ڳ���CO2����������ʵ��
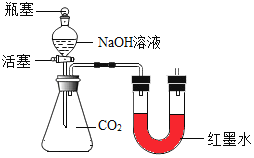
��ƿ���ͻ�����ʹNaOH��Һ���ٵ�����ƿ�У������رջ�����©����������Һʣ�ࣩ���۲쵽U���Ҳ�ĺ�īˮҺ��_____������ߡ��������͡����ƶ�������С����������ж�CO2��NaOH�����˷�Ӧ��������_____��
����˼�����ۣ�С����ΪС���ʵ�鷽�������ܣ�������_____��
����չʵ�飩��ȤС���ͬѧ��Ϊ��������������ʾ�����ַ�������һ��̽��������д����
ʵ�鷽�� | �������� | ���� | ʵ����� |
����һ | ȡС��ʵ�����ƿ�ڵ���Һ��������������CaCl2��Һ | _____ | CO2��NaOH�����˷�Ӧ |
������ | ȡС��ʵ�����ƿ�ڵ���Һ��������������_____�� | �����ݲ��� |
����˼������2��ͬѧ�Ǿ������ۣ���Ϊ����չʵ�飩��_____�������һ��������������Ȼ�����ܣ�������_____���û�ѧ����ʽ��ʾ����
���𰸡����� U�����Ҳ�Һ���½���˵����ƿ�ڵ�ѹǿ��С���Ӷ�˵�����������������̼�����˷�Ӧ ������̼����ˮҲ��ʹ��ƿ�ڵ�ѹǿ��С ������ɫ���� ϡ���� ����һ 2NaOH+CaCl2�TCa��OH��2��+2NaCl
��������
��ƿ���ͻ�����ʹNaOH��Һ���ٵ�����ƿ�У������رջ�����©����������Һʣ�ࣩ���۲쵽U���Ҳ�ĺ�īˮҺ�潵�ͣ�С����������ж�CO2��NaOH�����˷�Ӧ�������ǣ�U�����Ҳ�Һ���½���˵����ƿ�ڵ�ѹǿ��С���Ӷ�˵�����������������̼�����˷�Ӧ��
��˼�����ۣ�С����ΪС���ʵ�鷽�������ܣ������ǣ���ͨ������£�1���ˮ�ܽ�1�����CO2��������̼����ˮҲ��ʹ��ƿ�ڵ�ѹǿ��С��
��չʵ�飺����һ��ȡС��ʵ�����ƿ�ڵ���Һ������̼�������Ȼ��Ʒ�Ӧԭ����Na2CO3��CaCl2=2NaCl��CaCO3������������CaCl2��Һ��������ɫ������
��������ȡС��ʵ�����ƿ�ڵ���Һ������̼������ϡ���ᷴӦԭ����Na2CO3+2HCl=2NaCl+H2O+CO2������������ϡ���ᣬ�����ݲ�����CO2��NaOH�����˷�Ӧ��
��˼������2��ͬѧ�Ǿ������ۣ���Ϊ��չʵ��ķ���һ��Ȼ�����ܣ������ǣ�2NaOH+CaCl2�TCa��OH��2��+2NaCl��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�