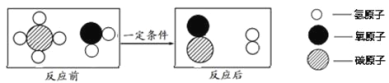
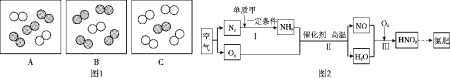
��Ŀ����
����Ŀ���������Ϲ㷺Ӧ�������������С�
��l���������Ƴ�����������������_________�ԣ�����硱����չ���������ġ����ұ�����ԭ����___________�����û�ѧ����ʽ��ʾ��
��2��С������ͼʾװ�ý��мס��ҶԱ�ʵ�飬̽���¶ȶ�CO��Fe2O3��Ӧ��Ӱ�죨�̶�װ���ԣ�
��������:Fe3O4�ڳ���������ϡ���ᷴӦ������ʽΪ��Fe3O4+8HCl=2FeCl3+FeCl2+4H2O������CuSO4��Һ��Ӧ��
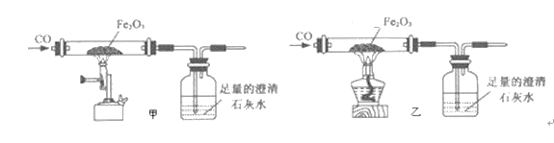
A.ʵ�鿪ʼʱӦ��ͨCO��Ŀ���ǣ�___________��
B.�ӻ����Ƕȿ��ǣ�ͼʾװ�û�Ӧ��ȡ�ĸĽ���ʩ��__________��
C.��ʵ������У�����ʯ��ˮ������ǣ������Ļ�ѧ��Ӧ����ʽΪ��_________����ȫ��Ӧ��������������Ϊ��ɫ��ĩ����������ֱ��������������������ʵ�飺
���� | ���� | �������� | �������� |
1 | ȡ��ɫ��ĩ���ô������� | �ܱ����� | �ܱ����� |
2 | ȡ��ɫ��ĩ������ϡ���� | ȫ���ܽ⣬�д������� | �����ݲ��� |
3 | ȡ��ɫ��ĩ����������CuSO4��Һ | �к�ɫ���ʲ��� | ���������� |
�ټ���ĺ�ɫ��ĩ��ϡ���ᷴӦ�Ļ�ѧ����ʽ��_________��
�ڸ���ʵ����������ʵ�鲣�����з����ķ�Ӧ����ʽΪ��__________��
�ۼס�������ʵ��˵���¶ȶ�CO��Fe2O3��Ӧ____________�����С���ȷ����Ӱ�졣
��3��С���Ի�ͭ��Cu �C Zn�Ͻ��е�Zn�ĺ���������̽������4.0g��ͭ��ĩ�ŵ�ʢ��10gϡ������ձ��У�ǡ����ȫ��Ӧ������ձ���ʣ�����ʵ�����Ϊ13.96g�� ��:
������������������_________g��
���������ǸúϽ��г�Cu��Zn����������ɷ֣������ԭCu �C Zn�Ͻ���Zn����������_______��(��������ȷ��С�����һλ)
���𰸡���չ 4Al+3O2=2Al2O3 �ž��Թ��ڿ��� ��װ��ĩ�˵��ܿڼ�һյȼ�ŵľƾ��� Ca(OH)2+CO2=CaCO3��+H2O Fe+2HCl=FeCl2+H2�� 3Fe2O3+CO![]() 2Fe3O4+CO2 �� 0.04 32.5%
2Fe3O4+CO2 �� 0.04 32.5%
��������
��1�������Ƴ���������������չ�ԣ�������չ�����ڿ����к�������Ӧ�������ܵ���������Ĥ���Ӷ���ֹ����һ������������4Al+3O2=2Al2O3��
��2��
A��ʵ�鿪ʼʱҪ��ͨһ����̼���ž��Թ��ڿ�������ֹһ����̼�Ϳ�����������ϼ���ʱ������ը�������ž��Թ��ڿ�����
B��һ����̼�ж����Ǵ�����Ⱦ�Ӧ����ʵ��װ��������β������װ�ã����Լ������ռ���Ҳ���Լ�һȼ�ŵľƾ��Ƶ㣬������װ��ĩ�˵��ܿڼ�һյȼ�ŵľƾ��ƣ�
C��������̼ʹ����ʯ��ˮ���������Ϊ������̼�ͳ���ʯ��ˮ��Ӧ����������ˮ��̼��Ƴ�����ˮ������Ca(OH)2+CO2=CaCO3��+H2O��
�����ݼ����ɫ��ĩ�ܱ�������������ɫ����ȫ���ܽ�������������ܺ�����ͭ��Һ��Ӧ��˵�����鷴Ӧ���ɵĺ�ɫ����ֻ����������ϡ���ᷴӦ�����Ȼ�����������������Fe+2HCl=FeCl2+H2����
����������ʵ������ɫ��ĩ�ܱ����������������ֲ����û���ϡ�����е�����Ҳ�����û�������ͭ�е�ͭ��˵����ɫ��ĩ������������Ҳ�����������ٽ����Ŀ�������ϣ������������ܺ�ϡ���ᷴӦ����û���������ɣ�����������Ҳ���ܺ�����ͭ��Ӧ�������������ɵĺ�ɫ����������������������3Fe2O3+CO![]() 2Fe3O4+CO2��
2Fe3O4+CO2��
�����Ϸ�����֪�¶���һ����̼��ԭ������ʵ������Ӱ�죬�����У�
��3��
����ͭ��ͭ����ϡ���ᷴӦ��ֻ��п��ϡ���ᷴӦ��������п����������Ӧǰ��ͭ��ϡ���Ṳ��4.0g+10g��=14g����Ӧ��ʣ����������ֻ��13.96g�����������غ㶨�ɿ�֪���ߵIJ�ֵ�������ɵ���������������������������=��14g-13.96g��=0.04g������0.04��
�����Ϸ�����֪������������Ϊ0.04g��ԭ��ͭ��п����������=![]() ������Ҫ�����ԭ��ͭ��п�����������ݻ�ѧ����ʽ���м��㣬��4.0g��ͭ��п������ΪX��
������Ҫ�����ԭ��ͭ��п�����������ݻ�ѧ����ʽ���м��㣬��4.0g��ͭ��п������ΪX��
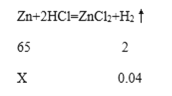
���X=1.3g �����������=![]() =32.5%������32.5%
=32.5%������32.5%

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�����Ŀ����ͼ��ֽ�����뼯��ƿ�У�����������˵������ȷ���� (�� )
ֽ�� | ����ƿ��ʢ�ŵ����� | ����ƿ��ֽ����ɫ�ı仯 | |
A | ��ɫʯ����ݺ�,���ɵ�ֽ�� | ������̼ | ֽ����Ϊ��ɫ |
B | ��ɫʯ����ݺ��ʪֽ�� | ������̼ | ֽ������ɫ��Ϊ��ɫ |
C | ��ɫʯ����ݺ��ʪֽ�� | �������� | ֽ������ɫ��Ϊ��ɫ |
D | ��̪��Һ���ݺ��ʪֽ�� | ����Ũ��ˮ | ֽ���ɺ�ɫ��Ϊ��ɫ |
A. A B. B C. C D. D
����Ŀ����ѧ��ȤС��Ϊ�ⶨһ��ʯ��ʯ��Ʒ��̼��Ƶ�����������ȡ��20gʯ��ʯ��Ʒ�����и������գ���Ʒ�г�̼����⣬����ɷָ��²��ֽ⡣̼��Ʒֽ�Ļ�ѧ����ʽΪCaCO3 == CaO + CO2��������ͬʱ����ʣ�������������±���ʾ��
���յ�ʱ�䣨min�� | ʣ������������g�� |
2 | 17.8 |
4 | 13.4 |
6 | 12.3 |
8 | 12.3 |
��1����ȫ��Ӧ�����ɶ�����̼������_____________��
��2����ʯ��ʯ��Ʒ��̼��Ƶ���������_______