��Ŀ����
����Ŀ������ijУͬѧ��ʵ������ȡ���岢��֤�����ijЩ���ʣ�����ͼ�ش����⣮
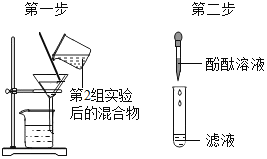
��1��д���������ƣ�a__��
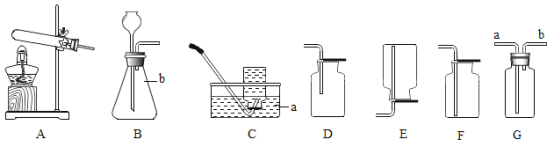
��2��ʵ�����ø��������ȡ�����Ļ�ѧ����ʽΪ__����Ҫ�ռ���Ϊ�������������ѡ�õ��ռ�װ����__������ĸ����
��3��ʵ������ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼�Ļ�ѧ����ʽ__����ѡ�õķ���װ����__������ĸ����
��4��ѡ��Eװ���ռ�����ʱ������ʵ�������ȷ����__��ѡ�����ֱ�ţ���
�ٷ�Ӧǰ��������ƿע��ˮ���ò���Ƭ��סƿ�ڣ�������ʢˮ��ˮ����
�ڿ�ʼ��Ӧ����������ð��ʱ��Ӧ���������ܿ����뼯��ƿ
���ռ����������ƿ���ϲ���Ƭ���Ƴ�ˮ�ۣ�
���𰸡���ƿ 2KMnO4![]() K2MnO4+MnO2+O2�� D CaCO3+2HCl=CaCl2+H2O+CO2�� A �٢�
K2MnO4+MnO2+O2�� D CaCO3+2HCl=CaCl2+H2O+CO2�� A �٢�
��������
��1��ͨ������������ָ���������ÿ�֪��a����ƿ�������ƿ��
��2����������ڼ��ȵ���������������ء��������̺���������ѧ����ʽΪ��2KMnO4![]() K2MnO4+MnO2+O2����Ҫ�ռ���Ϊ������������������ſ���������ѡ��D�����2KMnO4
K2MnO4+MnO2+O2����Ҫ�ռ���Ϊ������������������ſ���������ѡ��D�����2KMnO4![]() K2MnO4+MnO2+O2����D��
K2MnO4+MnO2+O2����D��
��3��̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2�����÷�Ӧ�ķ�Ӧ���ǹ����Һ�壬��Ӧ�����dz��£����Կ�ѡ�õķ���װ����A�����CaCO3+2HCl=CaCl2+H2O+CO2����A��
��4���ٷ�Ӧǰ��������ƿע��ˮ���ò���Ƭ��סƿ�ڣ�������ʢˮ��ˮ���У�����ȷ��
�ڿ�ʼ��Ӧ����������������ð��ʱ��Ӧ���������ܿ����뼯��ƿ���ʴ���
���ռ����������ƿ���ϲ���Ƭ���Ƴ�ˮ�ۣ�����ȷ����ѡ���٢ۡ�

����Ŀ��ijͬѧ���֣��ϸ�����ʵ���õ�NaOH��Һ�����˸�ƿ�ǣ����ڸ���Һ�Ƿ���ʣ�ͬѧ�ǿ�ʼʵ��̽����
��������裩
���루1��������Һû�б��ʣ�ΪNaOH��Һ��
���루2��������Һȫ�����ʣ�ΪNa2CO3��Һ��
���루3��������Һ���ֱ��ʣ�ΪNaOH��Na2CO3�Ļ����Һ��
���������ϣ�Na2CO3��Һ�ʼ���
����Ʒ��������������±�����ͬѧ̽�ֵ���Ʒ���
ʵ����� | ���ܳ��ֵ���������� | ͬѧ���� | |
��1�� |
| ����Һ��죬����루2�������� | ��2��ͬѧ��Ϊ���˷������۲���ȷ�������ǣ�__�� |
��2�� |
| ��������ɫ��������Ӧ����ʽ�ǣ�_____ ����루1���������� | ��3��ͬѧ��Ϊ���˷���������ȷ�����루2�����Dz��루3�������� |
��3�� |
| ����Һ����죬����루2������������Һ��죬����루3�������� | ��1��ͬѧ��Ϊ���������Ҳ�ܴﵽʵ��Ŀ�ģ����IJ��������ǣ�_____ |
��̽��ʵ�飩�ۺϸ�С��ķ�������ʵ�飮
�������뷴˼����1��NaOH��Һ���������_____��Ӧ�����ʣ�Ϊ��ֹ���ʣ����Ա���ʱҪ_____��
��2���ڶ���ʵ���У����������ķ���ʽΪ��_____��
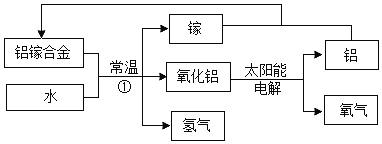
����Ŀ��(6��)�ڡ���ۡ��ۡ����š�֮�佨����ϵ����ѧϰ��ѧ��һ����Ҫ˼ά��ʽ����ͼ��Ԫ�����ڱ��в���Ԫ�ص�ԭ�ӽṹʾ��ͼ��������ѧ֪ʶ�ش��������⡣
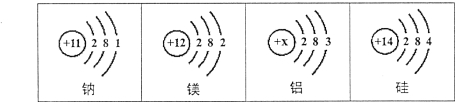
��1����ͼ����Ԫ���У����ڷǽ���Ԫ�ص��� (��Ԫ�ط���)��
��2��2��þ���ӵķ���Ϊ ����ԭ�ӵĺ˵����XΪ �����������Ļ�ѧʽΪ ��
��3�����ԭ��������ԭ����ʵ����֮����ڽ��ܵĹ�ϵ(���±�)�������±��ж�6.02��1023����ԭ�ӵ�����yΪ ��������±��еĹ��ɣ�
��
̼ | �� | �� | |
���ԭ������ | 12 | 16 | 23 |
6.02��1023��ԭ�ӵ����� | 12g | 16g | y |