��Ŀ����
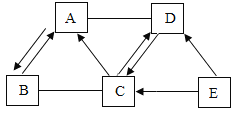
����Ŀ����ѧѧϰС���ͬѧ������ͼ��ʾ��װ�òⶨij����Ԫ��M�����ԭ��������
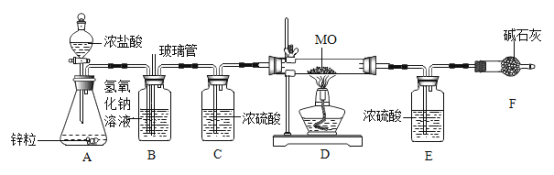
[�����ʵ��] ��ÿ����Ӧ��ֽ��У�
������װ�ã������װ�������ԣ�
�ڽ��ý���Ԫ�ص������MO������Ӳ�ʲ������У�����������Ϊ76.6g ��Ӳ�ʲ����ܵ�����Ϊ55.0g����
�۴�Һ©����������ƿ�еμ�Ũ���ᣬ��װ���ڿ����ž�����װ��E������Ϊ200.0g��
�ܵ�ȼ�ƾ��ƣ���D�й�����ȫ��Ӧ��ֹͣ���ȡ���D�в�������ȴ�����£��رշ�Һ©��������
�ݳ���װ��E������Ϊ205.4g��
[��������]
��1��д��Bװ���з�����Ӧ�Ļ�ѧ����ʽ____________��
��2��Cװ�õ�������_____________��
[����]
��3��������������㣬�ó�M�����ԭ������Ϊ________________���������1λС������
[������]
��4����D����Ʒδ��ȫ��Ӧ�����õ�M�����ԭ��������___________������ƫ��������ƫС��������Ӱ��������
[��չ̽��]
��5��ʵ���������ͬѧ���:�������ø�ʵ����֤ˮ������Ԫ�ص������ȣ����貹�������Ӧ��____װ�� ������ĸ����������
���𰸡�NaOH+HCl�TNaCl+H2O ����ˮ���� 56.0 ƫ�� D
��������
��1��Bװ�����������������Ȼ������壬������Ӧ�Ļ�ѧ����NaOH+HCl�TNaCl+H2O ��
��2��Ũ���������ˮ�ԣ�Cװ�õ�����������ˮ������
��3��205.4g-200.0g=5.4g��ˮ����Ԫ�ص�����Ϊ![]() ��MԪ������Ϊ76.6g-55.0g-4.8g=16.8g����M�����ԭ������Ϊx��x��16=16.8g��4.8g��x=56.0��
��MԪ������Ϊ76.6g-55.0g-4.8g=16.8g����M�����ԭ������Ϊx��x��16=16.8g��4.8g��x=56.0��
��4����D����Ʒδ��ȫ��Ӧ��ˮ������ƫС����Ԫ������ƫС�����õ�M�����ԭ��������ƫ��
��5��ʵ���������ͬѧ������������ø�ʵ����֤ˮ������Ԫ�ص������ȡ����貹�������Ӧ��Dװ�õ�����������Dװ�õ�������ó���Ԫ������������Eװ��������֪��ˮ�������������������Ԫ������������������Ԫ�ص������ȡ�

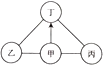
����Ŀ����ͼ�����кͷ�Ӧ���ճ������ũҵ���������Ź㷺��Ӧ�á���ͼ��ʾ���������������Һ������Ӧʱ�ձ�����Һ��pH�����Һ������ı仯������ص�ʵ�����������л�ȡ��Ϣ�ش���������:

(1)���ձ���ʢ�ŵ���_____��Һ��
������������Ϊ(18,7)�ĵ�����ʾ������______��
������������Ϊ( 15, 12)�ĵ��ʾ����Һ�е�����_____________(�û�ѧ�����ʾ)��
(2)С���������ʵ�鼸�����,���Ǹ���ʢ������������Һ�Լ�ƿ��ƿ���������С���������ʵ�鷽������������������Һ�Ƿ���ʡ�
ʵ�鷽�� | ʵ�鲽�� | ʵ������ | ʵ����� |
����һ | ȡ������Һ���Թ��У��μӼ���ϡ���ᡣ | û����������� | û�б��� |
������ | ȡ������Һ���Թ��У��μӼ����Ȼ�����Һ�� | _________________ | �Ѿ����� |
��д���������з�����Ӧ�Ļ�ѧ����ʽ_______________________________��
������Ϊ����һ��ʵ������Ƿ���ȷ?_______________(������ȷ����������ȷ��)����˵������___________________��
����Ŀ����ʵ��ⶨ�Ȼ��ƺ�������ڲ�ͬ�¶ȵ��ܽ���������±�������˵������ȷ���ǣ�������
�¶ȣ��� | 10 | 20 | 30 | 40 | 50 |
NaCl��g | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
KNO3��g | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 |
A.KNO3 ��NaCl���ܽ����ȵ��¶���20�桫30��֮��
B.�������ʵ��ܽ�ȶ����¶ȵ����߶�����
C.��20��ʱ��10gˮ�м���5gNaCl�ɵõ���������Ϊ33.3%��NaCl��Һ
D.�ֱ��������ʵ�100g������Һ��50�潵��10�棬KNO3�����ľ����