��Ŀ����
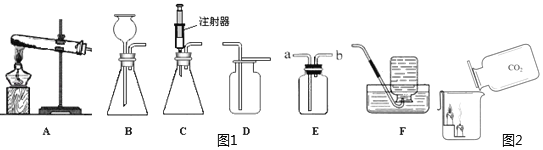
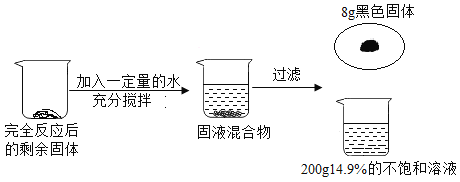
����Ŀ��С����ʵ���Ҽ���57g����غͶ������̵Ļ����һ��ʱ���ʣ����������Ϊ47.4g��С����������ʣ���������ȫ��Ӧ�����ռ���һЩ������Ϊ�˻��մ�������������ͼ��ʾʵ�飺
��ش��������⣺
��1��ʵ������з�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��2����һ�μ��Ⱥ��ռ�������������Ϊ______________________________________��
��3���г���������ܹ��μӷ�Ӧ�����������X�ı���ʽ_____________________��
��4��С�����μ��ȹ����У���������ص�������Ϊ____________________________��
��5���罫������Һ�Ƴ�������������Ϊ10%����Һ�������ˮ��������__________��
���𰸡�2KClO3![]() 2KCl + 3O2 �� 9.6g
2KCl + 3O2 �� 9.6g ![]() 1��1 98g
1��1 98g
��������
��1��������ڶ������̵Ĵ����������������Ȼ��غ���������ѧ����ʽΪ�� 2KClO3![]() 2KCl + 3O2 ����
2KCl + 3O2 ����
��2�������������������ǹ�̬�������ٵ���������һ�μ��Ⱥ��ռ�������������Ϊ��57g-47.4g=9.6�ˣ�
��3�����������֪����Ӧ���ɵ��Ȼ��ص�����=200g��14.9%=29.8g��
�������ܹ��μӷ�Ӧ�����������X
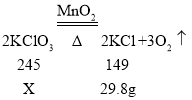
![]() X=49g
X=49g
��4�����һ�ηֽ������ص�����Ϊy��
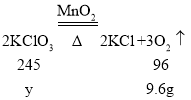
![]() y=24.5g��
y=24.5g��
С�����μ��ȹ����У���������ص�������=24.5g:��49g-24.5g��=1��1��
��5���������ˮ��������z��
��Һϡ�����У����ʵ��������䣬���У�
200g��14.9%=(200g+z)��10% z=98g���������ˮ��������98g��