��Ŀ����
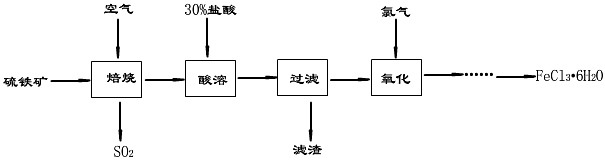
Ϋ���м���ʵʩũ�弯�й�ˮ���̣����й�ˮ�����˿��Ѵ�90%���ϡ�����ˮ��������������ɱ�������������Ȼ���������������ˮ������������Ҫ�ɷ�ΪFeS2��Ϊԭ���Ʊ��Ȼ������壨FeCl3��6H2O���Ĺ����������£�
�ش��������⣺
��1������������30%�����ᡰ���ܡ����պ�IJ�������Ҫ�ɷ��Ȼ�������д����Ӧ�ķ���ʽ_________________________________________________________��
��2�������������γ����꣬Σ���������������з�����ȥ��
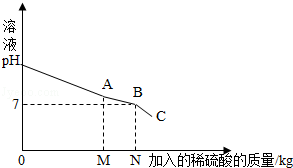
����1. ������������ķ���ͨ�백ˮ�����ն�������ˮ��pH__________7����д�����ڡ�����С�ڡ����ڡ�����
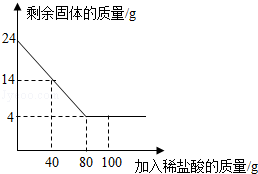
����2.������������ķ���ͨ��ʯ��ʯ������Һ�У��ڿ�����������������ƺͶ�����̼���Ӷ���ȥ��������д����Ӧ�ķ���ʽ______________________________________________________��
��3������ˮ���õ�ⱥ��ʳ��ˮ�Ʊ�������Cl2����Ӧ�ķ���ʽΪ
2NaCl+2H2O 2NaOH+ Cl2��+ H2��������Ҫ71t������������ˮ��������������Ҫ������10%�Ĵ��ζ��ٶ֣�ͬʱ�����ռ���ٶ֣�
2NaOH+ Cl2��+ H2��������Ҫ71t������������ˮ��������������Ҫ������10%�Ĵ��ζ��ٶ֣�ͬʱ�����ռ���ٶ֣�

�ش��������⣺
��1������������30%�����ᡰ���ܡ����պ�IJ�������Ҫ�ɷ��Ȼ�������д����Ӧ�ķ���ʽ_________________________________________________________��
��2�������������γ����꣬Σ���������������з�����ȥ��
����1. ������������ķ���ͨ�백ˮ�����ն�������ˮ��pH__________7����д�����ڡ�����С�ڡ����ڡ�����
����2.������������ķ���ͨ��ʯ��ʯ������Һ�У��ڿ�����������������ƺͶ�����̼���Ӷ���ȥ��������д����Ӧ�ķ���ʽ______________________________________________________��
��3������ˮ���õ�ⱥ��ʳ��ˮ�Ʊ�������Cl2����Ӧ�ķ���ʽΪ
2NaCl+2H2O
 2NaOH+ Cl2��+ H2��������Ҫ71t������������ˮ��������������Ҫ������10%�Ĵ��ζ��ٶ֣�ͬʱ�����ռ���ٶ֣�
2NaOH+ Cl2��+ H2��������Ҫ71t������������ˮ��������������Ҫ������10%�Ĵ��ζ��ٶ֣�ͬʱ�����ռ���ٶ֣��������ƣ������������ȣã죽�ƣ�ã������������ϣ����֣�
���������ڣ����֣������ӣ��������ã�ã���������=���ã�ӣ��������ã��������֣�
��3��80t
���������ڣ����֣������ӣ��������ã�ã���������=���ã�ӣ��������ã��������֣�
��3��80t
��1�������������ᷴӦ�Ļ�ѧ����ʽΪ���ƣ������������ȣã죽�ƣ�ã������������ϣ���2������������ķ���ͨ��ʯ��ʯ����Һ�еĻ�ѧ����ʽ���ݻ�ѧ����ʽ����д����д�����ɣ�ע�����������뷴Ӧ����3�����ݻ�ѧ����ʽ�����δ֪����������֪�����㼴�ɡ�
�������۽���裺�����Ȼ��Ƶ�����Ϊ���������ռ������Ϊ����
���Σ�ã죫�������� ���Σ�ϣȣ����������ã�����
���Σ�ϣȣ����������ã�����
������������������������������������������������
����������������������������������������������
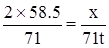 ���������������������������֣�
���������������������������֣�
 ���������������������������֣�
���������������������������֣�
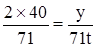 �����������������������������֣�
�����������������������������֣�
����Ҫ�����ʣ������Ĵ��Σ��������������ռ������Ϊ��������
�������۽���裺�����Ȼ��Ƶ�����Ϊ���������ռ������Ϊ����
���Σ�ã죫��������
 ���Σ�ϣȣ����������ã�����
���Σ�ϣȣ����������ã�����������������������������������������������������
����������������������������������������������
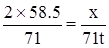 ���������������������������֣�
���������������������������֣� ���������������������������֣�
���������������������������֣�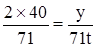 �����������������������������֣�
�����������������������������֣�����Ҫ�����ʣ������Ĵ��Σ��������������ռ������Ϊ��������

��ϰ��ϵ�д�
�����Ŀ