��Ŀ����
����Ŀ��θ��ƽ����Al��OH��3����С�մ�Ƭ����NaHCO3����θ�����Ƽ�����Ҫ��CaCO3��Mg��OH��2�����dz��õ��к�θ���ҩ�
��1��θ��ƽ��θ�ᷢ����Ӧ�Ļ�ѧ����ʽΪ��_____��
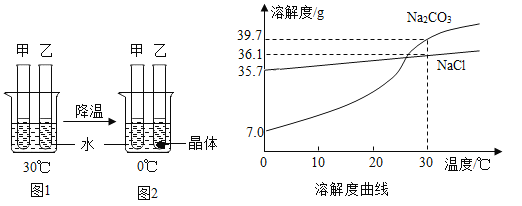
��2��Ϊ��̽����ҵС�մ����Ƿ�������NaCl���������ʵ�飺������С�մ�Ƭ���������ʾ�����Cl-��
ʵ�鲽�� | ʵ������ | ʵ����� |
��һ����ȡ1ƬС�մ����Թ��У���������ˮ����ܽ⣬�ٵμ�������ϡHNO3�� | ��_____���� | ˵��С�մ�Ƭ�к���NaHCO3�� |
�ڶ���������������Һ�еμ�����_____��Һ����д��ѧʽ�� | ��_____���� | ˵��С�մ�Ƭ�к���NaCl�� |
��3��θ�����Ƽ�ÿƬlg��ȡ10Ƭ���ձ��У��ټ���50gϡHCl���ձ���ʣ�����������ʱ��Ĺ�ϵ���±�������֪�������ʲ���ϡ���ᷴӦ��
ʱ��/s | 0 | 10 | 20 | 30 | 40 | 50 |
ʣ���������/g | 60 | 59.12 | 58.24 | 57.36 | 56.48 | 56.48 |
��ÿƬθ�����Ƽ���CaCO3����������_____��д��������̣���
���𰸡�Al(OH)3+3HCl=AlCl3+3H2O ���� AgNO3 ��ɫ���� 80%
��������
��1��θ�����Ҫ�ɷ������ᡣ���������������Ӧ�����Ȼ�����ˮ���ʴ�Ϊ��Al(OH)3+3HCl=AlCl3+3H2O��
��2��ȡ1ƬС�մ����Թ��У���������ˮ����ܽ⡣�ڵμ�������ϡ���ᣬϡ�����̼�����Ʒ�Ӧ���������ơ�ˮ�Ͷ�����̼���塣�������м���������������Һ����������Һ���Ȼ��Ʒ�Ӧ���������ƺ��Ȼ����������ʴ�Ϊ�����ݡ�AgNO3����ɫ������
��3����10Ƭ��Ʒ�к���̼��Ƶ�����Ϊx��
��Ӧ���ɶ�����̼������Ϊ��60g-56.48g=3.52g

���x=8g
������Ʒ��̼��Ƶ���������Ϊ��![]() ��
��