��Ŀ����
����Ŀ��ij��ѧ��ȤС����в�����������(FeC2O42H2O)�ֽ��ʵ��̽����
�����룩������������ֽ����� CO��CO2 �� H2O �������塣


��ʵ�鷽����������ͼװ�ý���ʵ��(�г�װ��δ����)��
���������ۣ�
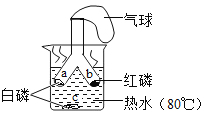
��1��ʵ�鿪ʼǰ��Ҫ�ȹ���һ��ʱ��� N2���ò�����Ŀ��Ϊ________________��
��2��C ������������Һ��������__________________��
��3��E �м�ʯ�ҵ�������__________________��
�����������
��4������
��֤���ֽ�����д��� CO ��������______________________��
��С����ΪӦ���� H װ�ã��� H װ��Ӧ����____________����װ��֮�䣬���۲� ��____________����֤����ˮ���ɣ�
��5����������(�ٶ�ÿһ����Ӧ������ȫ)��ȡ 3.6g �������������������ʵ�飬���װ��A Ӳ�ʲ������в���1.44g ��ɫ����FeO��װ��F ��Ӳ�ʲ����ܹ�����������0.32g���������������(FeC2O42H2O)�ֽ�õ��� CO ������Ϊ__________________��
����˼���ۣ�
��6���ӻ����Ƕȿ��ǣ�����ʵ��װ�õ�����ȱ����_______________��
��7��������ʾ��FeC2O42H2O ���ȷֽ�ʱ��������������¶ȱ仯����������ͼ��ʾ��д�����ȵ� 400�� ʱ��FeC2O42H2O ���ȷֽ�Ļ�ѧ����ʽ___________________��

����ͼ������3.6gFeC2O42H2O �ڳ��ڻ����г�ּ��ȣ����յõ�����ɫ���� 1.60g�� ������ʵĻ�ѧʽΪ____________���ɴˣ�����Ϊ���и�ʵ����Ҫע���������_____________��
���𰸡���װ���ڵĿ����ž�����ֹ������ը������еĶ�����̼��ˮ��������ʵ���� ���շ�Ӧ���ɵĶ�����̼ ����ˮ���� װ��F���ɺ�ɫ��Ϊ��ɫ������ʯ��ˮ����� AB ��ˮ����ͭ���� 0.56g ȱ��β������װ�� ![]() Fe2O3 FeC2O42H2O�ֽ�ʵ��Ӧ���ܱ������н���
Fe2O3 FeC2O42H2O�ֽ�ʵ��Ӧ���ܱ������н���
��������
�������ۣ���1��ʵ�鿪ʼǰ����Ҫ����һ��ʱ��ĵ�������װ���ڵĿ����ž�����Ϊ������������ֽ����� CO��CO2 �� H2O �������壬һ����̼���п�ȼ�ԣ�����һ�����Ŀ�����������ᷢ����ը��ͬʱ�����еĶ�����̼��ˮ�������ܻ�����������������װ���ڵĿ����ž�����ֹ������ը������еĶ�����̼��ˮ��������ʵ������
��2�������������뷴Ӧ���ɵĶ�����̼��Ӧ����̼���ƺ�ˮ����C ������������Һ�������ǣ����շ�Ӧ���ɵĶ�����̼��
��3����ʯ�Ҿ�����ˮ�ԣ���������ˮ��������E �м�ʯ�ҵ������ǣ�����ˮ������
���
��4����һ����̼��������ͭ�ڼ��ȵ������·�Ӧ����ͭ�Ͷ�����̼������ͭΪ��ɫ��ͭΪ��ɫ���ҷ�Ӧ���ɵĶ�����̼��ʹ����ʯ��ˮ����ǣ���֤���ֽ�����д��� CO �������ǣ�װ��F���ɺ�ɫ��Ϊ��ɫ������ʯ��ˮ����ǣ�
����ˮ����ͭ��ˮ�������ʸ�װ�ÿ���֤����ˮ���ɣ�ʵ����Ӧ����֤��ˮ�Ĵ��ڣ���Ϊ��������Һ��Я��ˮ����������ʵ��������Ӧ�������A��Bװ��֮�䣬��ˮ����ͭ������֤����ˮ���ɣ����AB����ˮ����ͭ������
��5��װ��F��Ӳ�ʲ����ܹ�����������0.32g��˵������ͭ��һ����̼�ڼ��ȵ������·�Ӧ������ͭ�Ͷ�����̼�����ٵ�������������ͭ����Ԫ�ص��������������������(FeC2O42H2O)�ֽ�õ��� CO ������Ϊx

![]() x=0.56g��
x=0.56g��
��˼���ۣ���6��һ����̼�ж���δ��Ӧ��һ����̼�ŷŵ������У���Ի��������Ⱦ����Ӧ����β������װ�ã����ȱ��β������װ�ã�
��7����ͼ��֪�����ȵ� 400�� ʱ�����ɹ��������Ϊ1.44g����ù���Ļ�ѧʽΪFemOn���ù�����������Ԫ�ص�����Ϊ��![]() ����Ԫ�ص�����Ϊ��1.44g-1.12g=0.32g��56m��16n=1.12g��0.32g��m��n=1:1���ʸù���Ļ�ѧʽΪ��FeO��������������ֽ������ CO��CO2 �� H2O �������壬�÷�Ӧ�Ļ�ѧ����ʽΪ��
����Ԫ�ص�����Ϊ��1.44g-1.12g=0.32g��56m��16n=1.12g��0.32g��m��n=1:1���ʸù���Ļ�ѧʽΪ��FeO��������������ֽ������ CO��CO2 �� H2O �������壬�÷�Ӧ�Ļ�ѧ����ʽΪ��![]() ��
��
3.6g FeC2O42H2O����Ԫ�ص�����Ϊ��![]() ���ú���ɫ��������Ԫ�ص�����Ϊ��1.60g-1.12g=0.48g����ú���ɫ���ʵĻ�ѧʽΪ��FeaOb��56a��16b=1.12g��0.48g��a��b=2:3���ú���ɫ����Ļ�ѧʽΪ��Fe2O3��
���ú���ɫ��������Ԫ�ص�����Ϊ��1.60g-1.12g=0.48g����ú���ɫ���ʵĻ�ѧʽΪ��FeaOb��56a��16b=1.12g��0.48g��a��b=2:3���ú���ɫ����Ļ�ѧʽΪ��Fe2O3��
�ɴ˿�֪��FeO������������Ӧ���������ʽ��и�ʵ��ʱ��Ӧ���ܱջ����н��С�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�ƵĴ��㡣�ס��ҡ������������������ʣ����ǵIJ��ֻ�ѧʽ����ʾ��ͼ�ֱ������±��С�
���� | �� | �� | �� | �� | �� |
��ʾ��ͼ |
|
|
|
|
|
��ѧʽ |
|
|
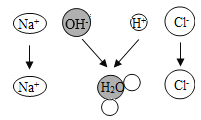
��1���÷��ű�ʾ2��������______�����������У��������������______![]() �û�ѧʽ��ʾ
�û�ѧʽ��ʾ![]() ��
��
��2����ҵ�����������ķ���֮һ�����üס������������ڸ��¡���ѹ�����ɱ��Ͷ������õ��ı������ֻ��漴�������ʷ�����Ӧ�����ɶ����졣ͨ����һϵ�з�Ӧ���μӷ�Ӧ�ļ͵õ��Ķ�������Ϊ______��