��Ŀ����
�ձ���װ��һ�������������ͭ�Ļ����Һ����֪����Һ�к�H2SO4������Ϊ9��8g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������10%��NaOH��Һ���õ�������������¼���£�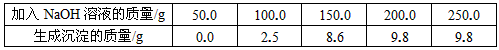
��1���õ�������������Ϊ g���û����Һ������ͭ������Ϊ g��
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�
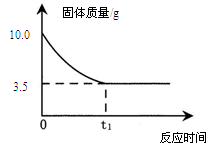
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߡ�
���������������1�����ϱ���¼�����ݿ�֪���տ�ʼ����NaOH��Һ����û�����ɳ�����ԭ����ԭ�ձ���װ��һ�������������ͭ�Ļ����Һ�������NaOH��Һ���Ⱥ����ᷴӦ��H2SO4 + 2NaOH=Na2SO4 + 2H2O��Ȼ������NaOH��Һ�IJ��ϼ��룬������NaOH��Һ������ͭ��Ӧ���ɳ�����2NaOH + CuSO4=Cu��OH��2�� + Na2SO4
����������NaOH��Һ�ļ������룬���ɵij�������Ҳ��Խ��Խ�����ձ���9��8g���䣬��ʾ��Ӧ����������ͭ�������꣬��������ͭ�ͳ���Cu��OH��2��������ϵ�������������ͭ������
��2��ͬ������ѧ��Ӧ��2NaOH + CuSO4=Cu��OH��2�� + Na2SO4�У�����NaOH�����Cu��OH��2��������ϵ��Ҳ������μӷ�Ӧ��NaOH���ʵ���������Ŀ��֪NaOH��Һ���������������������Ϳ�������μӷ�Ӧ��NaOH��Һ��������
��3����ͼ��һ��Ҫע�������㣺��ʼ�㣨��ʾij��Ӧ�Ŀ�ʼ����ת�۵㣨��ʾij��Ӧ�Ľ�����������ղŷ����ģ��տ�ʼ����NaOH��Һ����û�����ɳ�����ԭ����ԭ�ձ���װ��һ�������������ͭ�Ļ����Һ�������NaOH��Һ���Ⱥ����ᷴӦ��H2SO4 + 2NaOH=Na2SO4 + 2H2O����ԭ��Һ�к�H2SO4������Ϊ9��8g�����ݻ�ѧ����ʽ��NaOH��H2SO4��������ϵ���������Ҫ��9��8g H2SO4��ȫ��Ӧ����Ҫ��NaOH��������Ҳ����ͼ���е���ʼ�㣬����Ӧ����ʱ���ĵ�NaOH�������ڵڣ�2�����Ѿ��������ͼ���е�ת�۵㣬��ʱ��Һ������ͭҲ��ȫ�����ģ����Գ�������Ҳ�Ͳ��ٷ����仯
��2���⣺�������ᷴӦ��NaOH������Ϊx ����CuSO4��Ӧ��NaOH������Ϊy��
H2SO4+ 2NaOH��Na2SO4 + 2H2O �� CuSO4 + 2NaOH ��Cu��OH��2��+ Na2SO4
98 80 �� 80 �� 98
9��8g x �� y �� 9��8g 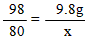
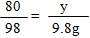
x = 8g y =8g
�ʲμӷ�Ӧ��NaOH��Һ��������Ϊ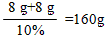
�����������вμӷ�Ӧ��NaOH��Һ����������160�ˡ�
���㣺���ݻ�ѧ����ʽ���м���

̼��̼���������������г��������ʣ�Ҳ�dz��л�ѧѧϰ����Ҫ���ݣ�
��1����ͼ �ǣ�����˵������ȷ������ ����
�ǣ�����˵������ȷ������ ����
| A����ԭ�ӵ�������Ϊ8 |
| B����ԭ���ڻ�ѧ��Ӧ����ʧȥ���� |
| C�����ǵؿ��к�������Ԫ�� |
| D����ԭ���������ӵĻ�ѧ���ʲ�ͬ |
��3��ʵ�������λͬѧ����ȡ������̼��
��С��ͬѧ��Ũ��Ϊ15%��ϡ�������״����ʯ��Ӧ��ȡ������̼��С��ͬѧ��Ũ��Ϊ8%��ϡ�������״����ʯ��Ӧ��ȡ������̼������С��ͬѧ�ռ�����ͬ���������̼���ٶȱ�Сͬѧ�죬�ɴ����ܵõ��Ľ������� ����
��Сƽͬѧ�ü���̼�����Ƶķ���Ҳ���Ƶ�CO2����֪̼���������ȷֽ�����̼���ơ�������̼��ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ�� ����
��4��С��������������Һ���Ȼ�����Һ��ϣ���������������һ��ʱ������ٴ�ȡ���ڷ��õ�����������Һ���л�ϣ���������˰�ɫ�����������û�ѧ����ʽ���Ϳ��ܵ�ԭ����ʾ��BaCO3�ǰ�ɫ��������ˮ��
��5��һ����̼��������Ȼ�������ƵĻ�ѧ���ʣ��绹ԭ�ԡ���ȼ�Եȣ���һ����̼ȴ��������Դ�ķ�չ���Ӱ�ȫ�ͻ����ĽǶ������������е���Ҫԭ����ʲô��
��6����ȡ5gʯ��ʯ�����ʲ��μӷ�Ӧ�������ձ��У������м�������ϡ���ᣬ����Ӧ���ɵ�����ȫ��ͨ������������ʯ��ˮ���ձ��У�����ȫ�������գ�����Ӧ����������ձ������ʵ�����������1.76g���Լ���ʯ��ʯ�к������ʵ�����������
 4FeO+CO2�����Լ���Ӧ��160gFe2O3��ĩ�м���ľ̿�۵�������
4FeO+CO2�����Լ���Ӧ��160gFe2O3��ĩ�м���ľ̿�۵�������