��Ŀ����
����Ŀ����ͼ��dz��н�����dz���Ҫ�Ļ����
(1)��ͼ�֮���ܷ����кͷ�Ӧ���÷�Ӧ��ʵ����________________��
(2)ʵ�鿼��ʱ����Ҫ��1%������������Һ100g���йز�����ͼ1��ʾ��
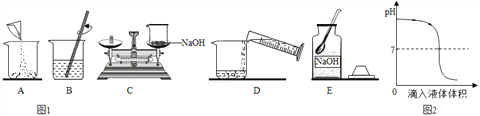
����ʵ����ȷ�IJ���˳����_____(����ĸ���ű�ʾ)��C�����������ԵĴ�����ȷ�IJ���Ӧ����______����ȡһ�������ˮʱ����Ҫѡ�õĺ��ʵ�������_____��
(3)��pH��ֽ�ⶨ�����Ƶ���Һ�����ȣ�д������IJ���������
��ʵ����չ����ͼ2������������Һ�������õμӷ�ʽ��Ӧʱ����ҺpH�������Һ����仯�����ߡ��μ�˳�������֣�a�ǽ�����������Һ���������У�b�ǽ������������������Һ�С�
���������жϣ��÷�Ӧ�ĵμ�˳����_______(����a������b��)��ijͬѧ�ڵμӹ����У����ⷢ���������ݲ����������ԭ��_____(дһ������)��
��ʵ�鷴˼��ij�ѱ��ʵ�����������Һ100g�������м���������������Ϊ7.3%��ϡ����100g��ǡ����ȫ��Ӧ�õ�������Һ�������ɸ�������Һ�ɵõ������������_____��
���𰸡� H+��OH-��ϳ�H2O ECADB ��߷�NaOH���ұ߷����� 100mL��Ͳ����ͷ�ι� b NaOH���տ�����CO2���� 11.7g
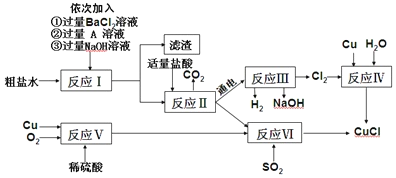
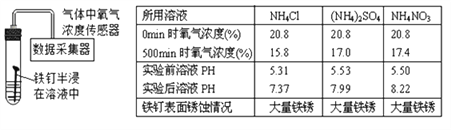
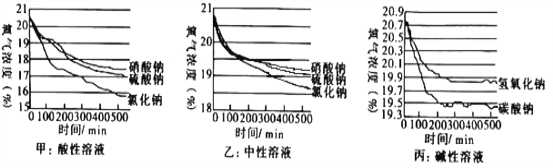
����������1������Ӧ��ʵ�������е�����������е����������ӷ�Ӧ����ˮ�Ĺ��̣�
��2��������Һ�IJ���Ϊ��������ҩƷ��ת��ҩƷ����ȡˮ���ܽ⣬��˳��ΪECADB��Cװ���е����������ŷ��ˣ���������ȡˮ�����Ϊ99ml����ѡ100ml����Ͳ��
��3����ͼ��֪���÷�Ӧǰ����Һ��pH����7 �����ǽ��������У���ѡb���緢��������ð���������������������Ʊ����ˣ����ɵ��Ȼ����е�������ȫ����ϡ�����ṩ��100g 7.3%��ϡ�������Ȼ��������Ϊ100g![]() 7.3%=7.3g�����������ӵ�����Ϊ7.3g
7.3%=7.3g�����������ӵ�����Ϊ7.3g![]() =7.1g����7.1g�������ӵ��Ȼ��Ƶ�����Ϊ7.1g
=7.1g����7.1g�������ӵ��Ȼ��Ƶ�����Ϊ7.1g![]() =11.7g
=11.7g

 �ظ���ʦ�㲦ϵ�д�
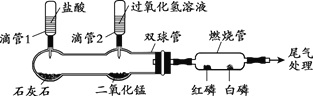
�ظ���ʦ�㲦ϵ�д�����Ŀ��������ͼװ�����A��B����ʵ�顣����ѡ1����������������𣬰�A�Ʒ֡�(�����Ż��Ϊ40�棬�����Ż��Ϊ240��)
A | B |
��ѹ�ι�1��һ��ʱ�����ȼ�չ���240�����ϡ� (1)˫����з�����Ӧ�Ļ�ѧ����ʽΪ_______�� (2)ȼ�չ��а��ͺ�����ȼ�գ���ԭ����___________�� | ��ѹ�ι�2��һ��ʱ�����ȼ�չ���80���� (1)˫����з�����Ӧ�Ļ�ѧ����ʽΪ___________�� (2)�ó���ȼ��ȼ����Ҫ�¶ȴﵽ�Ż��Ľ��ۣ���Ӧ��������___________�� |
| |