��Ŀ����
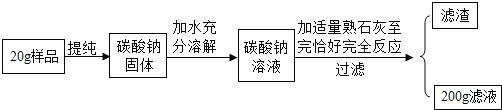
����Ŀ��(2017ʯ��ׯ������һģ)�����죬���������һ�����Сǿ���˰�װ��ǩ(��ͼ��ʾ)����������ʣ�̼���Ƶĺ�������Ҫ����Ϊ�ˣ�Сǿ�Ӱ�װ����ȡ�˲�����Ʒ������ѧУʵ���Һ���ʵ�飺����5.5 g��Ʒ�����ձ��У���ˮ�ܽ⣬�μ�ϡ���������ݲ��ٲ���Ϊֹ(ʵ���������¡����������ʲ���ϡ���ᷴӦ)��
��Ӧǰ | ��Ӧ�� | |||
ʵ�� ���� | ������Ʒ���ձ������� | ����ˮ������ | ����ϡ��������� | �ձ����ձ�����Һ������ |
55.5 g | 50 g | 50 g | 153.3 g | |
����㣺
��1�����ɶ�����̼��������________g��
��2��������Ʒ��̼���Ƶ����������Ƿ���ϰ�װ˵��Ҫ��
���𰸡���1��2.2��2��������
��������
�⣺�贿����Ʒ��̼���Ƶ�����Ϊx
Na2CO3��2HCl=== 2NaCl��H2O��CO2��
106 44
x 2.2 g
![]() ��
��![]()
x��5.3 g
������Ʒ��̼���Ƶ���������Ϊ![]() ��100%��96.4%<98%�������ϡ�
��100%��96.4%<98%�������ϡ�
�𣺴�����Ʒ��̼���Ƶ��������������ϰ�װ˵��Ҫ��

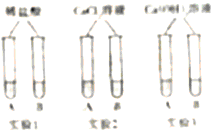
����Ŀ����ѧʵ����̾��������һ�����ķ�Һ������������������Ч��ֹˮ����Ⱦ����һ��ʵ����ϣ���ȤС���ͬѧ�������NaOH��Һ��Na2CO3��Һ�ļ��𣬲��Է�Һ�ɷ�չ��̽����
�һ
ͨ��С�������ͬѧ���������ͼ��ʾ������Сʵ�顣ʵ�������ɰ�ɫ�����Ļ�ѧ����ʽ��____________________(дһ��)��
���
Сѩ����֧�Թ��е�ʣ���ﵹ��һ���ྻ�Ĵ��ձ���(����ͼ)�� ��ֽ��衢���á��۲쵽�����ϲ�����ɫ��Һ���²��а�ɫ�������ɴ˿���ȷ�����ϲ���Һ��һ�������е�������__________________���Լ�һ�����е����ӡ�С��ͬѧ���ϲ���Һ�л����ܺ��е����ӽ���������̽����![]()
��������⣩�ϲ���Һ�л����ܺ���ʲô����?
����������裩�ϲ���Һ�л����ܺ���OH-��CO32-��Ca2+�е�һ�ֻ��֡�
������ʵ�飩
ʵ�鲽�� | ʵ������ | ʵ����� | |
����һ | ��ȡ������Һ���Թ��У��μ���ɫ��̪��Һ�ڼ����μ�ϡ���� | ����Һ��� �ڲ������� | ����OH- ����CO32-����Ca2+ |
������ | ȡ������Һ���Թ��У�____________(ָʾ������) | ��____________ ��___________ | ����CO32-����Ca2+ ����OH- |
����˼�����ۣ�
(1)С��ͬѧ�Է���һ������ɣ� ����������________________________��
(2)�����ۺϷ���������ȷ���ϲ���Һ�п϶����ڵ�������______________________��
(3)���ձ������ʹ��ˣ��������գ�����Һ�м�������_______________________���д��������ŷš�