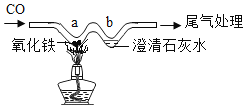
��Ŀ����
����Ŀ���á����ᡪ���ᱵ��������BaCl2��ų��ж�����H2S����ˮ��Һ�����ԣ�������NaOH����H2S��ȡNa2S���д���������ط�Ӧ�Ļ�ѧ����ʽ��H2S+2NaOH=Na2S+2H2O��
��Ŀ | ���ۣ�Ԫ/�֣� |
NaOH | 2500 |
Na2S | 5000 |
�������ã��Դ���1��H2S�ƣ� | 1114 |
��1����ij������ÿ��Ҫ����10��H2S��������NaOH���ٶ�_____��
��2���±�ΪH2S���չ��յĸ��������ɱ���
�����������Ϸ������Ӿ���Ч�濼�����ִ���H2S�ķ�����___��ѡ�ӯ�������𡱣��ġ�
���𰸡�23.5t ӯ��
��������
��1���⣺������ÿ��Ҫ����10��H2S����������NaOH����Ϊx��
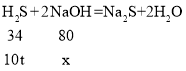
![]()
��ã�x��23.5t
��������NaOH��������23.5t��
��2���⣺��ÿ����һ��H2S�����������Ƶ�����Ϊy�����ɵ����Ƶ�����ΪZ��
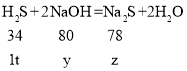
![]()
![]()
���y��2.35t��z��2.29t
ÿ����һ��������Ҫ2.35t�������ƣ����ĵķ��ã�![]() ���������Ƶ�������2.29t��������ã�
���������Ƶ�������2.29t��������ã�![]() ��11450Ԫ-5875Ԫ-1114Ԫ=4461Ԫ���Ӿ���Ч�濼�����ִ���H2S�ķ�����ӯ���ġ�
��11450Ԫ-5875Ԫ-1114Ԫ=4461Ԫ���Ӿ���Ч�濼�����ִ���H2S�ķ�����ӯ���ġ�

 ��ĩ1�����ʽ���������ϵ�д�
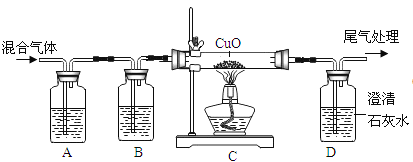
��ĩ1�����ʽ���������ϵ�д�����Ŀ����ѧ��ȤС��Ϊ�ⶨ����ʯ��̼��Ƶĺ���������ͼ��ʾ:��������ϡ������뵽20g����ʯ��(�����ɷֲ������ᷴӦ)���Ѳ�����CO2�������������ռ���Һ���գ�ͬʱ����Cƿ�ռ���Һ���ӵ�������������±���ʾ:
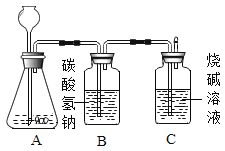
ʱ��/�� | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
C��������/C | 0 | 3.0 | 5.0 | 6.0 | 6.6 | x | 6.6 |
(1)��1���ϱ��У���10����ʱ��x=___;
(2)��2���������ʯ��Ʒ��̼��Ƶ���������(д���������) ___
(3)��3��������ͼ������ֽ�ϣ���ʱ��Ϊ�����꣬�Բ���CO2���������Ϊ�����꣬�����ܹ��������������������ʱ��仯���ɵĹ�ϵ����: ___;
��4��B����װҩƷΪ����̼��������Һ�����������տ��ܻӷ���HCl���壬����Ϊû��B�����������____.(�ƫ�ߡ���ƫ�͡�)��