��Ŀ����
����Ŀ�������� 100g ������������Ϊ 15%���Ȼ�����Һ����ش�
��1���������� 100g ������������Ϊ 15%���Ȼ�����Һ���裺�Ȼ���_____________g��ˮ____________g��
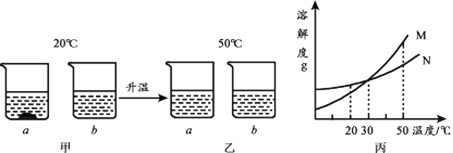
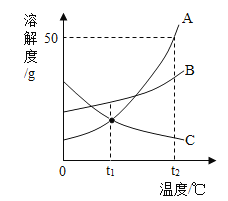
��2��������������ͼ��ʾ��
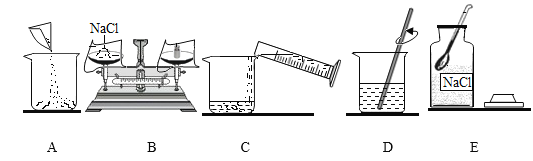
����ȷ�IJ���������____________������ĸ��
��C ������Ӧѡ��___________��10mL��50mL��100mL����Ͳ��ȡ����ˮ��
��D �����������������___________��
��������ϲ�����D����Ҫ�������Һװ��___________�У��Ǻ�___________,���ϱ�ǩ����ǩ������Ϊ___________,����õ��Լ��ŵ�____________�С�
��3�����д���������ܵ�����Һ������������С�� 15%���ǣ�����ţ�__________��
��B �����������ʳ�η��õߵ�����ʵ�����õ����룩
��D ��������Һ����
��A �����������
��C ����������������ˮ����
��4����ijͬѧ��� 200g ��������Ϊ 30%���Ȼ�����Һϡ��Ϊ 15%��ϡ��Һ����Ҫˮ��������________g��
���𰸡�15 85 EBACD 100mL ���ٹ��������ܽ� ϸ��ƿ ƿ�� 15% NaCl��Һ �Լ��� �٢� 200g
��������
��1���������� 100g ������������Ϊ 15%���Ȼ�����Һ���裺�Ȼ��Ƶ�����=100g��15%=15g����Ҫˮ������=100g-15g=85g��
��2������ȷ�IJ��������ǣ��ȼ��㡢Eȡҩ��B������Aת��ҩƷ��C��ˮ��D�����ܽ⣻
��ȷ����˳��Ϊ��EBACD��
��C ������ȡҺ�����ʱ��Ӧѡ�������Դ���ˮ���������Ҫˮ�����=![]() ��Ӧѡ100mL��Ͳ��ȡ����ˮ��
��Ӧѡ100mL��Ͳ��ȡ����ˮ��
��D ����ʹ�ò��������裬�����ǣ����ٹ��������ܽ⣻
��������ϲ�����D����Ҫ�������Һװ��ϸ��ƿ������ʢװҺ��ҩƷ�����У��Ǻ�ƿ��,���ϱ�ǩ����ǩ��������Ҫ��עŨ�ȣ���Һ���ƣ���ǩ��Ӧд�У�15% NaCl��Һ������õ��Լ��ŵ�ָ�����Լ����С�
��3�����д���������ܵ�����Һ������������С�� 15%���ǣ�
��B �����������ʳ�η��õߵ�����ʵ�����õ����룩�����³�ȡ�Ȼ�������ƫС��������������ƫС��
��D ��������Һ���䣬��Ӱ����������������
��A �����й������䣬�����Ȼ�������ƫС��������������ƫС��
��C ����������������ˮ�����������ܼ�����ƫС��������������ƫ��
��ѡ���٢ۣ�
��4����ijͬѧ��� 200g ��������Ϊ 30%���Ȼ�����Һϡ��Ϊ 15%��ϡ��Һ��ϡ��ǰ�����ʵ��������䣻��ϡ�ͺ���Һ������=![]() ����Ҫˮ������=400g-200g=200g��
����Ҫˮ������=400g-200g=200g��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�����Ŀ���������ڸ��ֽⷴӦ���ǣ�������
A.2H2O 2H2��+O2��B.CO+CuO
2H2��+O2��B.CO+CuO![]() Cu+CO2
Cu+CO2
C.NaOH+HCl��NaCl+H2OD.Fe+CuSO4=Cu+FeSO4
����Ŀ���á����ᡪ���ᱵ��������BaCl2��ų��ж�����H2S����ˮ��Һ�����ԣ�������NaOH����H2S��ȡNa2S���д���������ط�Ӧ�Ļ�ѧ����ʽ��H2S+2NaOH=Na2S+2H2O��
��Ŀ | ���ۣ�Ԫ/�֣� |
NaOH | 2500 |
Na2S | 5000 |
�������ã��Դ���1��H2S�ƣ� | 1114 |
��1����ij������ÿ��Ҫ����10��H2S��������NaOH���ٶ�_____��
��2���±�ΪH2S���չ��յĸ��������ɱ���
�����������Ϸ������Ӿ���Ч�濼�����ִ���H2S�ķ�����___��ѡ�ӯ�������𡱣��ġ�