��Ŀ����
����Ŀ��ijС���������С������ķ��䶯������������̽����

���������ϣ�
�ٻ��������Ҫ���÷���ԭ�����������������ɻ���·������������������ʹ���������ϵķ����������������������ڻ���ܵ�����������������֮��ʱ�����������档
�ڴ��ữѧ�������������ơ�
̽��һ���������Ļ�ѧ����
ͬѧ��������ͼ��ʾװ�ã�ѡ��ͬ�Ļ�ѧ��Ӧ���ڳ����½��л��ģ�ⷢ��ʵ�飬����������¡�
��� | ʵ����1 | ʵ����2 | ʵ����3 | |||||||||
H2O2��Һ | MnO2 ���� | ���о��� | ������Һ | Mg ���� | ���о��� | ���������� | ˮ ��� | ���о��� | ||||
�������� | ��� | �������� | ��� | |||||||||
1 | 7.5% | 100 mL | 1 g | 10.1 m | 15% | 100 mL | 1.2g | 10.6 m | 5 g | 100 mL | 0 | |
2 | 15% | 100 mL | 1 g | 12 m | 15% | 100 mL | 1.6g | 11.7 m | 10 g | 100 mL | 0 | |
3 | 30% | 100 mL | 1 g | 17 m | 15% | 100 mL | 2g | 12 .5m | 15 g | 100 mL | 0 | |
̽������ʵ����3ʧ�ܵ�ԭ��
����������裩�����ƹ����Ѿ����ʣ���ɷֿ����ǣ�
����1�� CaCO3 ����2��CaCO3��Ca(OH)2 ����3��CaCO3��Ca(OH)2��CaO
������ʵ�飩
�����.ȡ����������Ʒ���Թ��У�����һ������ˮ�����ִ����Թ���ڡ�
�����.���ˡ�
�����.ȡ�������μ�����ϡ���ᣬ�۲�����
�����.�Թ���ڲ����̣���̪��Һ����ɫ��������������
����������ۣ�
��1��̽��һ�У����ù���������Һ��Ϊ��������Ļ�ѧ��Ӧ����ʽΪ_________________��
��2��̽��һ�У�����ʵ����1��ʵ����2��ʵ�����ݿ�֪���������Ļ�ѧ������_______________�����йء�
��3��̽�����У�������ʵ��Ŀ����____________��
��4��̽�����У�ͨ��������ó���Һ�в����������ƣ����Ӧ�IJ�����������____________��
����˼�����ۣ�
��5��ͬѧ�����ۺ�һ����Ϊ��̽�����У����ݲ���������Ϳɵó�������Ʒ��һ��û��______��
��6��ʵ�����е���ʯ��Ӧ__________���档
���𰸡�2H2O2![]() 2H2O+O2�� ҩƷ��Ũ�Ⱥ�ҩƷ���� ֤����Ʒ���Ƿ���̼��� ȡ��Һ���μ���ɫ��̪��Һ����̪����ɫ CaO�� Ca(OH)2 �ܷ�
2H2O+O2�� ҩƷ��Ũ�Ⱥ�ҩƷ���� ֤����Ʒ���Ƿ���̼��� ȡ��Һ���μ���ɫ��̪��Һ����̪����ɫ CaO�� Ca(OH)2 �ܷ�
��������
[���������]
��1�����������ڶ������������������·ֽ�����������ˮ����ѧ��Ӧ����ʽΪ2H2O2![]() 2H2O+O2����
2H2O+O2����
��2��̽��һ�У�����ʵ����1��ʵ����2��ʵ�����ݿ�֪���������Ļ�ѧ������ҩƷ��Ũ�Ⱥ�ҩƷ���������йء�
��3��̼������ᷴӦ���ɶ�����̼���壬̽�����У�������ʵ��Ŀ����֤����Ʒ���Ƿ���̼��ƣ���������ݲ�������֤����Ʒ�к���̼��ơ�
��4��̽�����У�ͨ��������ó���Һ�в����������ƣ����Ӧ�IJ�����������ȡ��Һ���μ���ɫ��̪��Һ����̪����ɫ��
[��˼������]
��5�������Թ���ڲ����̣���̪��Һ����ɫ���ɵó�������Ʒ��һ��û��CaO�� Ca(OH)2��
��6����ʯ����������е�ˮ������Ӧ�����������ƶ����ʣ�ʵ�����е���ʯ��Ӧ�ܷⱣ�档

 ��������һ���þ�ϵ�д�
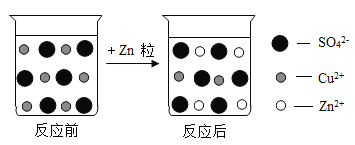
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ij��ѧ��ȤС��ͬѧ�˽�������Cl2���ǻ���ɫ���壬����ˮ��Һ����ˮ����ͬѧ�ǰ���ɫʯ����Һ�ӵ���ˮ�У�������Һ�ȱ�죬����ɫ��
���������ϣ�
��1����������ˮ��������C12���Ӵ�������Һ�У�������ˮ��ӦC12+H2O=HCl+HClO��
��2�������ᣨHCl����һ�����ᣬ����ǿ�����ԣ������ֽ⡣�䷴Ӧ����ʽΪ��2HClO![]() 2HCl+O2��
2HCl+O2��
�����������ʹ��ɫʯ����Һ������������ˮ�е������______��
��������⣩ʹ��ɫʯ����Һ��ɫ��������ʲô�أ�
��������룩
����1����ˮ�е�ˮʹ��ɫʯ����ɫ��
����2����ˮ�е�______ʹ��ɫʯ����ɫ��
����3����ˮ�е�����ʹ��ɫʯ����ɫ��
����4����ˮ�еĴ�����ʹ��ɫʯ����ɫ��
��ʵ��̽����ȡ�Ķ���ʯ����ҺȾ����ɫ�ĸ����ֽ����������ʵ�顣
ʵ����� | ʵ������ | ʵ����� |
����һ��ֽ����ˮ | ��û�����Ա仯 | ����1 ______ |
���ڶ���ֽ��ֱ�ӷ���ʢ�и���� ______ ����ƿ | ��û�����Ա仯 | ����2������ |
������ֽ������ϡ���� | ����죬�� ______ | ����3������ |
�����Ķ�ֽ������ˮ�� ______ | ���ȱ�죬����ɫ | ����4 ______ |
����չ̽����ʵ�����б�����ˮ�ķ�����______��
����˼�����ۣ�
��ȤС��ͬѧ�����̽�������е���һ������ʹ��ɫʯ����Һ��죬�������ʵ�鷽����ȡ�����������Թ��У����뼸����ɫʯ����Һ�����õι������μ�����������Һ�������Ͻ��裬����ɫ��ʧ��˵��ˮ���Ӻ�______���ѧ���ţ�����ʹʯ���졣
�����������ʵ�鷽��̽����֤��
ʵ����� | ʵ������ | ʵ����� |
______ | ______ | ______ |
����Ŀ������ʵ����Ʋ��ܴﵽ���Ӧʵ��Ŀ�ĵ���( )
ѡ�� | ʵ�� | ���� | ���� |
A |
| ��Ƭ�������������ݣ�п˿�����н϶����� | ˵������п�Ļ����� Fe<Zn |
B |
| ����ڿ�����ȼ�շ������ĵ���ɫ���棬�������з�������������ɫ���� | ˵�������������ȼ�ո���ʢ |
C |
| ���Թ���û���������� ���Թ����������� | ˵����������Ҫˮ |
D |
| �Թ��а���ȼ�գ���ˮ�а��ײ�ȼ�� | ˵��ȼ����Ҫ���� |
A. AB. BC. CD. D
����Ŀ��ʵ��С��ͬѧ��֤��ȼ��ȼ�յ���������������ʵ�顣
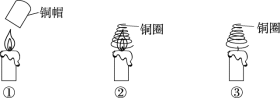
��� | ʵ����� | ʵ������ |
�� | ͭñ���Ⱥ������������������ | ����Ϩ�� |
�� | ͭȦ���Ⱥ������������������ | �������ȼ�� |
�� | ͭȦ�����ȣ�ֱ��������������� | ����Ϩ�� |
��1����֤��ȼ��ȼ����ҪO2��������______��
��2���Ա�ʵ��ںۣ͢����Եó���ȼ��ȼ�յ�������________��