��Ŀ����
����Ŀ���±���Ca(OH)2��NaOH���ܽ�����ݡ���ش��������⣺
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��/g | Ca(OH)2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 |
NaOH | 31 | 91 | 111 | 129 | 313 | 336 | |
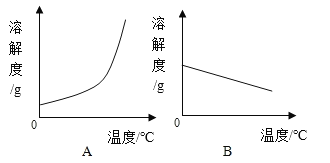
��1�������ϱ����ݣ�����Ca(OH)2��NaOH���ܽ�����ߣ���ͼ���ܱ�ʾNaOH�ܽ�����ߵ���_____����A��B����
��2��Ҫ���һƿ�ӽ����͵�Ca(OH)2��Һ��ɱ�����Һ�������ʩ�У�
�ټ����������ƣ��������¶ȣ��۽����¶ȣ��ܼ���ˮ��������ˮ���ٻָ���ԭ�¶ȣ�������ʯ�ҡ����д�ʩ��ȷ����_____��
A �ڢܢ� B �ۢ� C �٢ۢݢ� D �٢ڢݢ�
��3��20��ʱ��191g����NaOH��Һ������10gˮ���ٽ��µ�20����������NaOH���������Ϊ_____��
��4������20��ʱCa(OH)2�ı�����Һ������Һ���������м���һ����CaO��õ�����Һ������Һ������ʱ��Һ�����ʵ�����������_____�ף�����������������������������
��5������60��ʱ��Ca(OH)2��NaOH�������ʵı�����Һ����Ҫ�õ��ϴ�����NaOH���壬Ӧ��ȡ������������_____��
���𰸡�A D 9.1g �� ���½ᾧ������
��������
��1���ɱ������ݿ�֪��NaOH���ܽ�����¶ȵ����߶�����ѡA��
��2��Ca(OH)2���ܽ�����¶ȵ����߶���С���ټ����������ƣ���ʹ��������Һ��Ϊ������Һ���������¶ȣ��ܽ�ȼ�С����ʹ��������Һ��Ϊ������Һ���۽����¶ȣ��ܽ�������ܱ�Ϊ������Һ���ܼ���ˮ����Һ�Dz�������Һ��������ˮ���ٻָ���ԭ�¶ȣ���ʹ��������Һ��Ϊ������Һ��������ʯ�ң���ʯ����ˮ��Ӧ�����������ƣ���ʹ��������Һ��Ϊ������Һ����ѡD��
��3��20��ʱ��NaOH���ܽ����91g����191g����NaOH��Һ�к���100gˮ������10gˮ���ٽ��µ�20�棬������NaOH���������Ϊ![]() =9.1g��
=9.1g��
��4������20��ʱCa(OH)2�ı�����Һ������Һ���������м���һ����CaO����������ˮ��Ӧ�����������ƣ����ڷ�Ӧ���ȣ�Һ����¶����ߣ��������Ƶ��ܽ�ȼ�С����������������С����ʱ��Һ�����ʵ���������Ϊ���ң��ס�
��5������Ca(OH)2���ܽ�����¶ȵĽ��Ͷ�����NaOH���ܽ�����¶ȵĽ��Ͷ���С������60��ʱ��Ca(OH)2��NaOH�������ʵı�����Һ�������¶�ʱ��NaOH��Һ������NaOH���壬Ca(OH)2��Һ��Ϊ��������Һ������������Ȼ����ˣ��ܵõ��ϴ�����NaOH���塣

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�����Ŀ�������л�����������ij�������ʣ�Ϊ�ⶨ����������۵�����������ijС����������²���:��ȡ7.7g����Ȼ��80 gϡ������Ĵμ���ʢװ�����������У���ü���ϡ���������������������������ϵ���±���( �����ʲ��뵥������Ӧ)
��һ�� | �ڶ��� | ������ | ���Ĵ� | |
����ϡ���������/ g | 20 | 20 | 20 | 20 |
�����������ۼ�����/g | 0.05 | 0.15 | 0.20 | m |
��1������m��ֵΪ______�������μ��������________(������������δ��")��Ӧ�ꡣ
��2���������������۵����������Ƕ���?(д��������̣������ȷ��0.1%)
��3����������ݺͼ��������������л�����Ӧ�����ߡ�
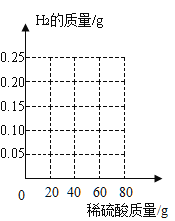
��4����һ�μ���ϡ����ʱ������������ֻ��0.05g ��ԭ�������________
����Ŀ����һ���������Ҵ���C2H6O������������һ����յ���������ȼ����÷�Ӧǰ������ʵ��������±��������ж���ȷ���� ( )
���� | �Ҵ� | ���� | ˮ | ������̼ | X |
��Ӧǰ����/g | 23 | 40 | 0 | 0 | 0 |
��Ӧ������/g | 0 | 0 | 27 | 22 | a |
A.����a��ֵΪ15
B.����X�Ǹ÷�Ӧ�Ĵ���
C.����X�п��ܺ�����Ԫ��
D.����ʼʱ����8g����������X����
����Ŀ��Ϊ�ⶨijNaCl��Na2CO3�����������ɣ�С��ͬѧȡ16 g�û��������ձ��У�����μ���ϡ���ᣨÿ�μ���ϡ���������Ϊ25g��������Ӧ��ȫ�õ������������ϵ��
����ϡ����Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� |
�ձ�����Ӧ�������������/g | 122.2 | 146.1 | 170.0 | 193.9 | 218.9 |
��������ݼ��㣺
��1��������ϡ��������������ǡ����ȫ��Ӧʱ������ ��CO2��
��2��������ϡ��������������ǡ����ȫ��Ӧʱ��������Һ�����������������������ȷ��0.1��