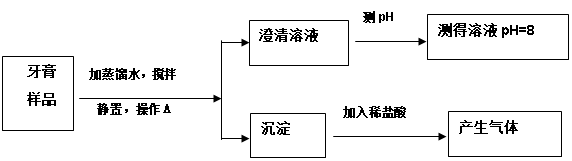
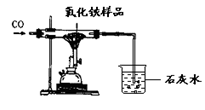
��Ŀ����
������ij��ȤС��ͬѧ����3��Сʵ�顣����ʵ�����ݻش��������⣨�����Ĺ̶�װ���Ѿ�ʡ��)��

��1��������Ϊ�������̣����Ӽ���װ�ã�______________(��ء�K1���͡�K2���IJ���)���ӷ�Һ©������м�������˫��ˮ����ʵ�����֤����ȼ��ȼ����Ҫ�������Ӵ������з�����Ӧ�Ļ�ѧ����ʽΪ____________________��
��2��������Ϊˮ������Ϊ��ɫ��Һ�����Ӽױ�����K1���ر�K2���ӷ�Һ©������м���ijҩƷ����ֻ������Һ�ɺ�ɫ��Ϊ��ɫ��д�����з�����Ӧ�Ļ�ѧ����ʽ______��
��3��������Ϊ������̼��K1��K2�رգ������Ӽ����ӷ�Һ©������м��������ij���ʯ��ˮ��һ��ʱ���K1������ʵ������м��й۲쵽������______________________��

��1��������Ϊ�������̣����Ӽ���װ�ã�______________(��ء�K1���͡�K2���IJ���)���ӷ�Һ©������м�������˫��ˮ����ʵ�����֤����ȼ��ȼ����Ҫ�������Ӵ������з�����Ӧ�Ļ�ѧ����ʽΪ____________________��
��2��������Ϊˮ������Ϊ��ɫ��Һ�����Ӽױ�����K1���ر�K2���ӷ�Һ©������м���ijҩƷ����ֻ������Һ�ɺ�ɫ��Ϊ��ɫ��д�����з�����Ӧ�Ļ�ѧ����ʽ______��
��3��������Ϊ������̼��K1��K2�رգ������Ӽ����ӷ�Һ©������м��������ij���ʯ��ˮ��һ��ʱ���K1������ʵ������м��й۲쵽������______________________��
|
 2H2O2 2H2O + O2��
2H2O2 2H2O + O2����2��2NaOH + H2SO4 = Na2SO4 + 2H2O
��3������ʯ��ˮ����ǣ���K1����Һ�嵹����ף�������ɫ���ݣ����Ǽ��٣���ʧ��������Һ�����붡��
�����������1��˫��ˮ�ڶ������̵Ĵ������·ֽ⣬����ˮ���������ʷ�Ӧ�Ļ�ѧ����ʽΪ2H2O2
 2H2O + O2����������˫��ˮ������¶˵ĵ����ܿڻᱻҺ�⣬��������ܶȶ���С���������Ĺܿ��ݳ�������ر�K1,��K2��
2H2O + O2����������˫��ˮ������¶˵ĵ����ܿڻᱻҺ�⣬��������ܶȶ���С���������Ĺܿ��ݳ�������ر�K1,��K2����2�����ݡ�ֻ����������Һ�ɺ�ɫ��Ϊ��ɫ����˵��������ҺԭΪ���з�̪�ļ�Һ�������������ڼ��е��ᣬ���к��˱��еļ�ʷ�̪��Ϊ��ɫ���Ҹ�����кͷ�Ӧ�����������ٸ������⣬�����Ӽױ�����K1���ر�K2���ӷ�Һ©������м���ijҩƷ����ֻ������Һ�ɺ�ɫ��Ϊ��ɫ����˵�����е���������ѹ�������£����¶˵ĵ����ܿڽ�����ģ�������ԭ��ˮ���ɴ˿�֪���ӷ�Һ©������м������Ũ���ᣬ��ˮ����ʹ�����������ͣ�ѹǿ���Ӷ�������ѹ����������еļ�Һ��Ӧ����2NaOH + H2SO4 = Na2SO4 + 2H2O��
��3��������Ϊ������̼��K1��K2�رգ������Ӽ����ӷ�Һ©������м��������ij���ʯ��ˮ�����߷�Ӧ����̼��Ƴ������ʿɼ�����ʯ��ˮ����ǣ�һ��ʱ���K1�����ڼ���������٣�ѹǿ��С�����ڴ���ѹ�������£�����ϡ����ᱻ������ף���֮ǰ��Ӧ���ɵ�̼��Ƴ�����Ӧ�������Ȼ��ơ�ˮ�Ͷ�����̼���壬�ʿɼ�����ɫ���ݲ��������Ǽ��١�
������������֤��ʵ��̽����Ҫ������ʵ����ʻ�仯���ɣ����ݸ�����ʵ����Ʒ���������ʵ�顢������̽������ͨ���۲졢��¼�ͷ�����ʵ����������֤�����ʵ����ʻ�仯���ɵȡ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ