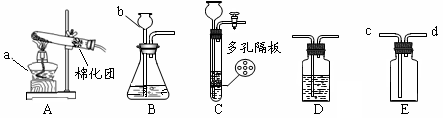
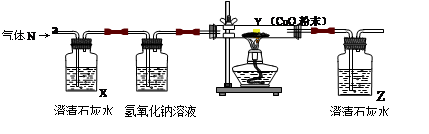
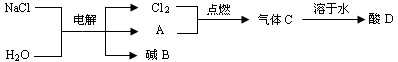��Ŀ����
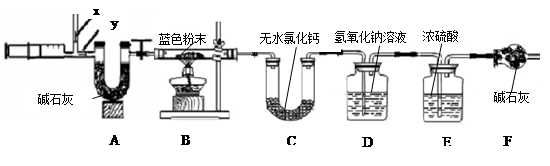
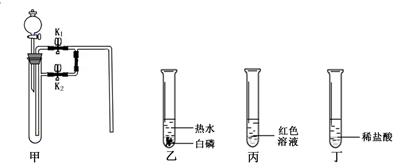
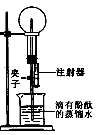
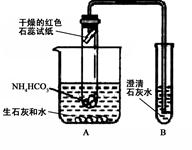
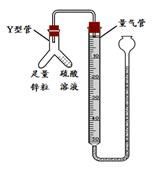
ͬѧ��������ѧʵ���ң����⿴����һ������г�ġ�������������ͼ�����ɴˣ�������ͬѧ�ǵ�̽��������

��1��ͬѧ��ȡ��ƿ��������Һ �μ�ϡ���ᣬ����ð���ݣ�˵��ҩƷ�ѱ��ʣ����ʷ�Ӧ�Ļ�ѧ����ʽΪ�� ��
�����в��롿С��IJ��룺NaOH ��Һ���ֱ��ʣ�
��IJ��룺 ��
��ʵ��̽����С���������ʵ������֤�Լ��IJ��룬������±���
������IJ�����ȷ������С���ʵ�鷽������ʵ�飬����۲������ ��
��ʵ�鷴˼����1����������1.BaCl 2��Һ 2.Ca(NO3)2��Һ 3.Ca(OH)2��Һ4.Ba(OH)2��Һ�� �������С��ʵ����CaCl 2��Һ���� (�����)�� ��
��2��С��ڶ��εμӵ��Լ�����ָʾ���⣬�������� �����
����չӦ�á�����NaOH ��Һ�ķ����� ��

��1��ͬѧ��ȡ��ƿ��������Һ �μ�ϡ���ᣬ����ð���ݣ�˵��ҩƷ�ѱ��ʣ����ʷ�Ӧ�Ļ�ѧ����ʽΪ�� ��
�����в��롿С��IJ��룺NaOH ��Һ���ֱ��ʣ�
��IJ��룺 ��
��ʵ��̽����С���������ʵ������֤�Լ��IJ��룬������±���
| ̽��Ŀ�� | ̽������ | Ԥ������ |
| ������Һ�е�CO32- | �٣�ȡ������Һ���Թ��У��μ�������CaCl2�Լ� | |
| ֤����Һ���д�NaOH | �ڣ���ʵ��ٹ��˺�������Һ�еμӷ�̪��Һ | |
��ʵ�鷴˼����1����������1.BaCl 2��Һ 2.Ca(NO3)2��Һ 3.Ca(OH)2��Һ4.Ba(OH)2��Һ�� �������С��ʵ����CaCl 2��Һ���� (�����)�� ��
��2��С��ڶ��εμӵ��Լ�����ָʾ���⣬�������� �����
����չӦ�á�����NaOH ��Һ�ķ����� ��
�� 2NaOH + CO2=Na2CO3 + H2O��NaOH ��Һȫ������
���ְ�ɫ��������Һû�б��
��ʵ�鷴˼�� (1) 3��4 (2) CuSO4��Һ�ȣ�����չӦ�á��ܷⱣ��
| ̽��Ŀ�� | ̽������ | Ԥ������ |
| | | ���ְ�ɫ���� |
| | | ��Һ��� |
��ʵ�鷴˼�� (1) 3��4 (2) CuSO4��Һ�ȣ�����չӦ�á��ܷⱣ��
�����������1�������������Ϳ����еĶ�����̼��Ӧ����̼���ƺ�ˮ���ҵIJ�����NaOH��Һȫ�����ʣ�Ϊ����֤�Ƿ���ȫ���ʣ�����ȡ������Һ���Թ��У��μ�������CaCl2�Լ�����ȫ��ȥ̼���ƣ�����Һ�м����̪��Һ�����˵�����������ƣ��Dz��ֱ��ʣ����������ȫ�����ʡ�
����������һ���dz����͵�̽���⣬��Ŀ����֪ʶ��û��ѧ������Ϊİ���������ص㿼��ķ�Ӧ��˼�룬װ�õȣ�����������Ŀ��Ҫ���£���ϸ���⼴�ɡ�

��ϰ��ϵ�д�
�����Ŀ
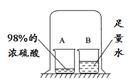
 CuSO4 �� 5H2O����
CuSO4 �� 5H2O����