��Ŀ����
����Ŀ����ѧ����������������ء�

��1��ͼ1������ʳ���У����ṩ����ά���ص���_____������ĸ��ţ���
��2���ӵ�������������ָ_____������Ԫ����������������������һ��ȱ�⣬���ܻ��еļ�����_____��������״���״���������������������
��3����������������ˮ����������_____������ʳ��������ʳ��ˮ������
��4�������е�����������ˮ�����γ���Һ����_____������ĸ��ţ���
A ������ B ʳ�� C ��� D ����
��5��ϴ�Ӳ;�ʱ��������ϴ�Ӽ������кܺõ�ϴ��Ч����ԭ����ϴ�Ӽ�������_____���á�
��6�����쳣��������ˮ���м��������Ҷ���֮��Ļ�������Է�ֹˮ���е�ˮ�������Ϊ�Ҷ�������ˮ��ʹ��Һ�����̵�_____��������������������������
��7����ͼ2��ʾ��������Ϩ����һ�����͵ļ��������Ʒ��������Ϩ���Ӵ�������![]() ���ը�����ͷŵķ�ĩ������ȼ�����ϣ�ͬʱ�ų�����ȼ���壬ʹ����Ϩ��������Ϩ�������ԭ����_____������ĸ��ţ���
���ը�����ͷŵķ�ĩ������ȼ�����ϣ�ͬʱ�ų�����ȼ���壬ʹ����Ϩ��������Ϩ�������ԭ����_____������ĸ��ţ���
A �����ȼ�� B ʹȼ�������������� C ����ȼ������Ż��
���𰸡�A Ԫ�� ��״���״� ʳ�� BD �黯 ���� �黯
��������
��1��ƻ���к��д���ά���أ�ţ���и��������ʣ�ʳ�����и�����֬�����������࣬��ѡ��A��
��2���ӵ�������������ָ��Ԫ�أ�ȱ�ٵ�Ԫ�أ����ü�״���״��Ԫ�أ���״���״�
��3��ˮ������Ҫ�ɷ���̼��ƺ�������þ��ʳ���д�����Ժ�̼��ƺ�������þ��Ӧ�Ӷ���ȥˮ�������ʳ�ף�
��4�������ͺ���۶�������ˮ�������γ���Һ��ʳ�κͰ��ǿ�������ˮ�γ���Һ����ѡ��BD��
��5��ϴ�Ӽ��������黯���ã����͵η�ɢ������ϸС��Һ�α�ˮ���ߣ�����黯��
��6�����̵�Խ��Խ������������������Ҷ���֮��Ļ�������Է�ֹˮ���е�ˮ�����˵�����̵㽵���ˣ�������ͣ�
��7��������Ϩ���Ӵ�������![]() ���ը�����ͷŵķ�ĩ������ȼ�����ϣ�ͬʱ�ų�����ȼ���壬�����������������ﵽ����Ч������ѡ��B��
���ը�����ͷŵķ�ĩ������ȼ�����ϣ�ͬʱ�ų�����ȼ���壬�����������������ﵽ����Ч������ѡ��B��

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
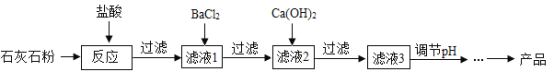
���ѵ����Ԫ��ĩ���100��ϵ�д�����Ŀ��ͬѧ��������ʵ����ʱ������һ����ɫ��ĩ״ҩƷ����ʦ˵��ҩƷ���Ȼ��ơ�̼��ơ����������е�������ɣ�ͬѧ�ǶԴ˺ܺ��棬���Ƕ������ɫ��ĩ�ijɷֽ�������̽����
���������룩����һ��_______ �������CaCO3��NaOH ��������NaCl��NaOH
�����ʵ�飩
ʵ�鲽�� | ʵ������ | ʵ����� |
��1��ȡһ������ɫ��ĩ���ձ��У�������ˮ������ | ________ | ������������ |
��2��ȡ������1����������Һ���Թ��У������е����Ȼ�ͭ��Һ | ________ | ����������� |
��3����ȡ������1����������Һ���Թ��У������еμӹ�����____��Һ | ������ɫ���� | ����һ���� |
д�����裨3���з�����Ӧ�Ļ�ѧ����ʽ____��
����չ���죩��βⶨ�ð�ɫ��ĩ��̼��Ƶ����������أ�ijͬѧ����̼�������ϡ���ᷴӦ����������̼���ⶨ�ð�ɫ��ĩ��̼��Ƶ�������������������װ�òⶨ������̼��������
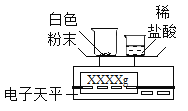
��1����С�ձ��е�����ϡ����ּ��μ��뵽���ձ��в����Ͻ��裬�жϰ�ɫ��ĩ��̼�����ȫ��Ӧ��ʵ��������_____��
��2����֪��Ӧǰ��������[m�����ձ�����ɫ��ĩ����m��С�ձ���ϡ���ᣩ]��Ҫ��������CO2�����������ٻ���Ҫ��������____������ţ���
A m��С�ձ��� B m�����ձ��� C m�����ձ�����Ӧ��ʣ���
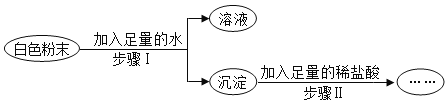
����Ŀ���ס��ҡ����������ʵ�ת����ϵ��ͼ��ʾ����������ʾ��Ӧ����һ��ʵ��(�������ʺͷ�Ӧ������ʡ��)������ѡ���в��ܰ�ͼʾת����ϵʵ�ֵ���
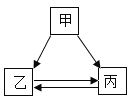
ѡ�� | �� | �� | �� |
A | H2SO4 | H2O | H2 |
B | NaOH | NaCl | NaNO3 |
C | C | CO2 | CO |
D | Ca (OH) 2 | CaCl2 | CaCO3 |
A.AB.BC.CD.D