��Ŀ����
����Ŀ��ij��ȤС��ͨ��þ��ϡ���ᷴӦ̽��Ӱ�췴Ӧ���ʵ����أ�þ��������ȣ�þ������״һ����ϡ����������������ʵ�������
ʵ���� | ������������� | þ����̬ | ��Һ��ʼ�¶�/�� |
�� | 3% | ��ĩ | 30 |
�� | 3% | ��״ | 20 |
�� | 6% | ��ĩ | 20 |
�� | 6% | ��״ | 20 |
��1��þ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ______��
��2��������ʵ������ռ���250mL����Ϊ����Ҫ�Ƚϸ��鷴Ӧ���ʣ�������ʵ�黹��Ҫ�����������______��
��3��ʵ��______��______����ʵ���ţ����о������Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�졣
��4���ⶨ�ڲ�ͬʱ�������������������ݣ����Ƴ�ͼ1����ʵ��ڶ�Ӧ�����߿�����______������ţ���
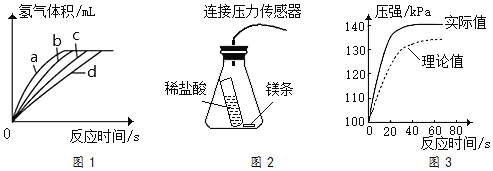
��5������ͼ2ѹ������������ʵ�飬�õ�ͼ3��ƿ�ڵ�ѹǿ��ʱ��仯������ͼ������ʵ���õ�ʵ��ֵ������ֵƫ����������ܵ�ԭ��______��
���𰸡�Mg+2HCl=MgCl2+H2�� �ռ�250mL������Ҫ��ʱ�� �� �� d ϡ�����þ��Ӧ���ȣ������������ͣ�ѹǿ����
��������
��1��þ��ϡ���ᷴӦ�����Ȼ�þ����������Ӧ�Ļ�ѧ����ʽ����Mg+2HCl�TMgCl2+H2����
��2��ʵ�黹��Ҫ������������ռ�250mL������Ҫ��ʱ�䣬�����ռ�250mL������Ҫ��ʱ�䣻
��3��ʵ��ں͢����о������Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�죬������Ϊ�ڡ�����þ����״���������¶ȶ���ȣ�ֻ������Ũ�Ȳ�ͬ������ڣ�����ܣ�
��4��ʵ�����У�����Ũ����С���¶���ͣ�þ������Ӵ������С����˷�Ӧ������������Ӧ�����߿�����d������d��
��5��ʵ���õ�ʵ��ֵ������ֵƫ���ܵ�ԭ����ϡ�����þ��Ӧ���ȣ������������ͣ�ѹǿ������ϡ�����þ��Ӧ���ȣ������������ͣ�ѹǿ����

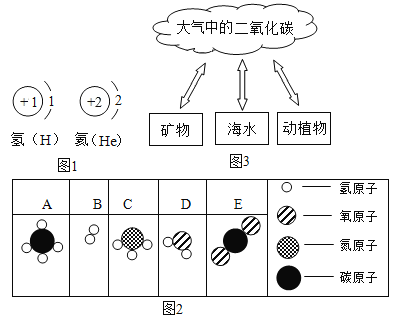
 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�����Ŀ��Ԫ�����ڱ��������ڵ�����Ԫ�ص��й����������
Ԫ������ | �� | þ | �� | �� | �� | �� | �� |
�˵���� | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
�������ϼ� | +1 | +2 | +3 | -4��+4 | -3��+5 | -2��+4��+6 | -1��+7 |
�����ĵ����� | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
��1���û�ѧ���ű�ʾ��
������ԭ��______��������______����Ԫ�ص��������������______��
��2����ԭ�ӽṹʾ��ͼ��ͼ ��ʾ��X����ֵΪ______��
��ʾ��X����ֵΪ______��
����n����ֵ�����ƶ��ڻ�ѧ�仯�����У���ԭ������______���ʧȥ���õ��������ӡ�
��3���۲����ݱ������ܽᡰ����Ԫ�ء�������۵Ĺ�����______��