��Ŀ����
����Ŀ��ij�������д����ᴿʵ�飬�������ͼ����ش�
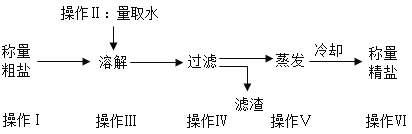
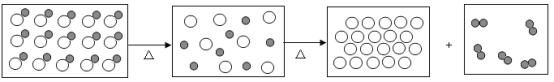
��1��ʵ���ҳ���������������õ�����ƽ������ֱ����ʾ���ƹ�����������ڷų���ֽ���ձ�����Ҫ����____________����������У��������������ȥƤ������
��2��������I��V��V�ж��õ��IJ��������� ____________���������ڲ���IV�е�������_________.
��3�������ᴿʵ�鲽��϶ࡢ�����������������̴����ᴿʵ��ʱ��IJ�������________
A ������� B �ܽ�ʱ���һ����ˮ
��4������V������Ϊ��ֹ�������е�Һ�ηɽ���Ӧ��ȡ�Ĵ�ʩ��___________�����������г��ִ�������ʱ��Ӧ___________������________��ˮ�����ɡ�
��5��ijͬѧ�����������Һ��Ȼ���ǣ�������ֽ�����������⣬������д��һ�������Һ���ǵ�ԭ��________
��6�����»�����������ܽ�-�������������з������_______
A ʳ�κͰ��� B ʳ�κ�ϸɳ C ʳ�κ�ζ�� D ���ͺ�ˮ
��7����С��IJ��ʼ��������£�
��� | 1 | 2 | 3 | 4 |
���� | 75% | 83% | 88% | 72% |
��ʦ�Բ��ʵ����� | ƫ�� | ƫ�� | ƫ�� | ƫ�� |
���и���ͬѧ����ɲ���ƫ��ƫ�͵�ԭ���������ȷ����__________�����ţ�.
A.��1�飺�ձ����ʳ��δ�ܽ���Ϳ�ʼ����
B.��2�飺����ʱ���й������ʷɽ�
C.��3�飺��Һ���ǾͿ�ʼ����
D.��4�飺���������л���ˮ��
���𰸡�ȥƤ ������ ���� A �ò��������裬ʹ���Ⱦ��ȣ���ֹҺ�ηɽ� ֹͣ���� ���� ����ҺҺ��߶ȸ�����ֽ��Ե�������𰸺������ɣ� B AC
��������
��1��ʵ���ҳ���������������õ�����ƽ������ֱ����ʾ���ƹ�����������ڷų���ֽ���ձ�����Ҫ����ȥƤ������
��2�������������ж��õ��IJ��������Dz��������������ڲ��� IV�е�������������
��3�������ᴿʵ�鲽��϶ࡢ����������������Ρ��ܽ�ʱ��ֽ���������̴����ᴿʵ��ʱ�䣬��ѡA��
��4������ V��������ֹ�������е�Һ�ηɽ���Ӧ�ò��������裻���������г��ֶ�������ʱ��ֹͣ���ȣ��������Ƚ�ˮ�����ɣ�
��5��ijͬѧ�����������Һ��Ȼ���ǣ���Һ���ǵ�ԭ������ֽ��������©����Һ�������ֽ��Ե��
��6�����á��ܽ�-���ˡ��������з�����ǹ̡�Һ����ʳ�κ�ϸɳ��ϸɳ��������ˮ��ʳ��������ˮ�������á��ܽ�-���ˡ��������з��룬��ѡB��
��7������ƫ�ߡ�ƫ�͵�ԭ���ǣ�A����1�飺�ձ����ʳ��δ�ܽ���Ϳ�ʼ���ˣ�D����4�飺����ʱ�������л�����ˮ�֡���ѡAC��

����Ŀ����ͼij��ȤС���KClO3�ֽⷴӦ�Ĵ��������о�������ͬ�ļ��������£���ɱ���ʵ�飺
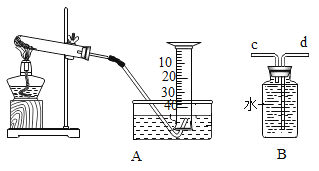
��� | KClO3����/g | ���� | ��������/g | �ռ�50mLO2����ʱ��/S |
ʵ��1 | 5 | ------ | ----- | 171 |
ʵ��2 | 5 | MnO2 | 0.5 | 49 |
ʵ��3 | 5 | Fe2O3 | 0.5 | 58 |
ʵ��4 | 5 | KCl | 0.5 | 154 |
��1�����ʵ��1��Ŀ��________________��
��2����������3�ִ����Ĵ�Ч����ѵ���__________________��
��3��д��ʵ��3�ķ�Ӧ���ű���ʽ_________________________��
��4����ʵ��1��4��֪��KCl _____�������������������������á�ά�ּ����������䣬��ʵ��1�ټ����ռ�50mLO2������ʱ����������171s������ԭ��_______________��
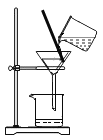
��5����Ƚ�KClO3�ֽⷴӦ�в�ͬ������Ч�������˲����ռ�50mLO2����ʱ���⣬�����Բ�����ͬʱ����_______________���ռ�װ�ÿ��Ը�����B��ʾ����Ҫ����һ�ֲ�������____________�������______________����c��d����������ƿ��ˮδװ����____________����������������������Ӱ��ʵ������
����Ŀ�������±��ش����⡣
�¶ȣ����� | 20 | 40 | 50 | 60 | 80 | |
�ܽ�ȣ�g/100g ˮ�� | NaCl | 36.0 | 36.6 | 37.0 | 37.3 | 38.4 |
NH4Cl | 37.2 | 45.8 | 50.4 | 55.2 | 65.6 | |
KNO3 | 31.6 | 63.9 | 85.5 | 110 | 169 | |
��1��20��ʱ�����������ܽ�ȴӴ�С��˳����__________��
��2��������к����������Ȼ������ʣ��ɲ���________���ᴿ����ء�
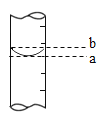
��3����Ͳ�ľֲ�ʾ��ͼ����ͼ������ȡˮʱӦ����____���ߣ�ѡ����a������b�������ж�����____���ߣ�ѡ����a������b������Ӧ�Ķ����ϴ�
��4��A��80������120 gˮ��KNO3��Һ���������²������õ�102 gKNO3���塣
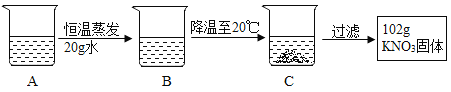
��.A��ҺΪ___________��ѡ��������������������������Һ��
��.�����Ϲ��̵ķ���������ȷ����__________��ѡ���ţ���
a.A��B�Ĺ����У���������û�иı�
b.B���������ܼ���������Ϊ169�U100
c.��ʼ����KNO3������¶���60����80��֮��
d.��Һ����������222 g