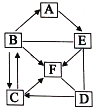
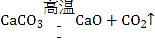��Ŀ����
����Ŀ����ͼij��ȤС���KClO3�ֽⷴӦ�Ĵ��������о�������ͬ�ļ��������£���ɱ���ʵ�飺
��� | KClO3����/g | ���� | ��������/g | �ռ�50mLO2����ʱ��/S |
ʵ��1 | 5 | ------ | ----- | 171 |
ʵ��2 | 5 | MnO2 | 0.5 | 49 |
ʵ��3 | 5 | Fe2O3 | 0.5 | 58 |
ʵ��4 | 5 | KCl | 0.5 | 154 |
��1�����ʵ��1��Ŀ��________________��
��2����������3�ִ����Ĵ�Ч����ѵ���__________________��
��3��д��ʵ��3�ķ�Ӧ���ű���ʽ_________________________��
��4����ʵ��1��4��֪��KCl _____�������������������������á�ά�ּ����������䣬��ʵ��1�ټ����ռ�50mLO2������ʱ����������171s������ԭ��_______________��
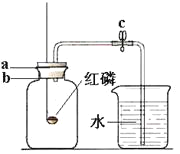
��5����Ƚ�KClO3�ֽⷴӦ�в�ͬ������Ч�������˲����ռ�50mLO2����ʱ���⣬�����Բ�����ͬʱ����_______________���ռ�װ�ÿ��Ը�����B��ʾ����Ҫ����һ�ֲ�������____________�������______________����c��d����������ƿ��ˮδװ����____________����������������������Ӱ��ʵ������
���𰸡������� MnO2 KClO3![]() KCl+ O2 �� ���ŷ�Ӧ�Ľ��У����ɵĴ���KClԽ��Խ�� ������� ��Ͳ c ����
KCl+ O2 �� ���ŷ�Ӧ�Ľ��У����ɵĴ���KClԽ��Խ�� ������� ��Ͳ c ����
��������
��1���ɼ���ʵ��Աȿ�֪�����ʵ��1��Ŀ���������ա���������ա�
��2�����ݱ�������3�ִ������Ƚ��ռ�50mLO2����ʱ��ij��̣�MnO2��ʱ����̣��ʴ�Ч����ѵ���MnO2�����MnO2��
��3��ʵ��3�Ǽ�������ع��壬�����������������÷�Ӧ���ű���ʽKClO3![]() KCl+ O2�����KClO3
KCl+ O2�����KClO3![]() KCl+ O2��
KCl+ O2��
��4����ʵ��1��4��֪��KCl Ҳ���Լӿ췴Ӧ���ʣ������Ĵ����á�ά�ּ����������䣬��ʵ��1�ټ����ռ�50mLO2������ʱ����������171s����Ϊ���ŷ�Ӧ�Ľ��У����ɵĴ���KClԽ��Խ�࣬���Ȼ������д����ã���˷�Ӧ�����ʱ������̡�����У����ŷ�Ӧ�Ľ��У����ɵĴ���KClԽ��Խ�ࡣ
��5����Ƚ�KClO3�ֽⷴӦ�в�ͬ������Ч�������˲����ռ�50mLO2����ʱ���⣬�����Բ�����ͬʱ�����ռ���������������ռ�װ�ÿ��Ը�����B��ʾ����Ҫ��������Ͳ����ȡ�ų�ˮ������������������������Ӧ��c���룬��ѹˮ����ˮ��������ƿ��������Ͳ��������ƿ��ˮδװ��������Ӱ��ʵ��������Ϊ���뼯��ƿ��������٣��ͻ��ų�����ˮ����������������Ͳ��c�����ᡣ

 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�����Ŀ��ij�������д����ᴿʵ�飬�������ͼ����ش�
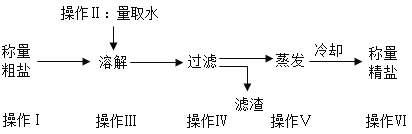
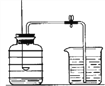
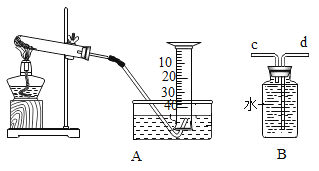
��1��ʵ���ҳ���������������õ�����ƽ������ֱ����ʾ���ƹ�����������ڷų���ֽ���ձ�����Ҫ����____________����������У��������������ȥƤ������
��2��������I��V��V�ж��õ��IJ��������� ____________���������ڲ���IV�е�������_________.
��3�������ᴿʵ�鲽��϶ࡢ�����������������̴����ᴿʵ��ʱ��IJ�������________
A ������� B �ܽ�ʱ���һ����ˮ
��4������V������Ϊ��ֹ�������е�Һ�ηɽ���Ӧ��ȡ�Ĵ�ʩ��___________�����������г��ִ�������ʱ��Ӧ___________������________��ˮ�����ɡ�
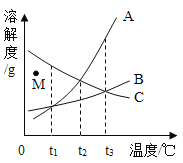
��5��ijͬѧ�����������Һ��Ȼ���ǣ�������ֽ�����������⣬������д��һ�������Һ���ǵ�ԭ��________
��6�����»�����������ܽ�-�������������з������_______
A ʳ�κͰ��� B ʳ�κ�ϸɳ C ʳ�κ�ζ�� D ���ͺ�ˮ
��7����С��IJ��ʼ��������£�
��� | 1 | 2 | 3 | 4 |
���� | 75% | 83% | 88% | 72% |
��ʦ�Բ��ʵ����� | ƫ�� | ƫ�� | ƫ�� | ƫ�� |
���и���ͬѧ����ɲ���ƫ��ƫ�͵�ԭ���������ȷ����__________�����ţ�.
A.��1�飺�ձ����ʳ��δ�ܽ���Ϳ�ʼ����
B.��2�飺����ʱ���й������ʷɽ�
C.��3�飺��Һ���ǾͿ�ʼ����
D.��4�飺���������л���ˮ��