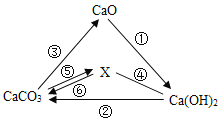
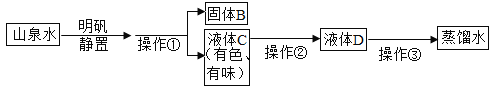
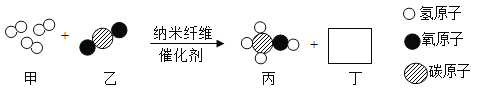
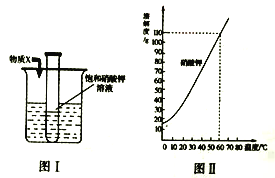
��Ŀ����
����Ŀ����ͼ����������κ�ˮ�ķ�Ӧ���кͷ�Ӧ��ij��ѧ��ȤС��ͬѧ���������к�����������Һ���ķ�Ӧ����̽����
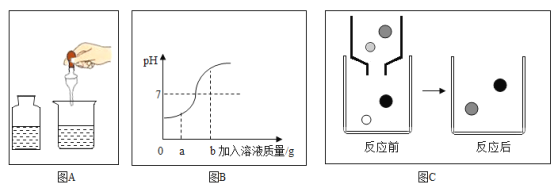
��1��С�������������к�����������Һ����ʵ��ʱ�������Ӧ��������Һ��pH�仯��ͼ��ʾ��
�ٸ÷�Ӧ�Ļ�ѧ����ʽΪ��___________________��
�ڸ�ʵ������ǽ�_________________�μӵ���һ����Һ�С�
�۵�������Һ������Ϊagʱ��������Һ�е��������У�д���ţ�_______________��
�ܵ�������Һ������Ϊbgʱ����������Һ��Һ�е�����ɫʯ����Һ����ɫʯ����Һ��_______ɫ��
��2��С�������������к�����������Һ����ʵ��ʱ��������������Һ��μ�����ǰ���˼���ָʾ�����������жϸ��кͷ�Ӧ���еij̶ȣ���������������Һ������Խ���̽����
��̽��Ŀ�ģ�̽��������Һ������ԡ�
��������룩������Һ���ܳ����ԣ�Ҳ���ܳ�____________�ԣ������ܳʼ��ԡ�
��ʵ����֤��
ʵ����� | ʵ������ | ���� |
���Թ�ȡ����Һ1-2mL������1-2����ɫ��̪��Һ���� | ��ɫ��̪��Һ��________ɫ | ��Һ�ʼ��� |
��ɫ��̪��Һ����ɫ | ��Һ��_________�� |
�������뷴˼��
������Һ�ʼ��ԣ�����Һ��ʹ��ʼ��Ե�������_________��д���ţ���
������ɫ��̪��Һ����ɫ��Ϊ�˽�һ��ȷ����Һ������ԣ�С����������·�����
����һ��ȡ��������þ��������____________����������ԣ�������������������ԡ�
��������ȡ��������һö������������������۲쵽������ʧ����Һ��ɻ�ɫ������ȡ��Һ�����ԣ���Ӧ�Ļ�ѧ����ʽΪ____________��������������������ԡ�
���𰸡�NaOH+HCl�TNaCl+H2O ����������Һ H+��Na+ �� �� �� ���Ի����� OH- ���ݲ��� Fe2O3+6HCl�T2FeCl3+3H2O
��������
��1���Լ�����Һ��ʹ��̪��Һ���ɫ����ʹʯ����Һ����ɫ����������Һ����ʹ��̪��Һ��ɫ����ʹʯ����Һ���ɫ�������Բ���ʹʯ����Һ����̪��Һ��ɫ��
��2��������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ������������Ӧ�����Ȼ�����ˮ��
��1�����������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��NaOH+HCl�TNaCl+H2O��
�ڹ�������ҺpH������ʵ������ǽ�����������Һ�μӵ���һ����Һ�С�
�������������Һ��
�۵�������Һ������Ϊagʱ��������Һ�����������ͬʱ�����Ȼ��ƣ���Һ�е���������H+��Na+��
���H+��Na+��
�۵�������Һ������Ϊbgʱ������������Һ��������Һ�Լ��ԣ���������Һ��Һ�е�����ɫʯ����Һ����ɫʯ����Һ����ɫ��
�������
��2��[�������]
������Һ���ܳ����ԣ�Ҳ���ܳ����ԣ������ܳʼ��ԡ�
�����
[ʵ����֤]
���Թ�ȡ����Һ1-2mL������1-2����ɫ��̪��Һ���������ɫ��̪��Һ���ɫ��˵����Һ�Լ��ԣ�
��ɫ��̪��Һ����ɫ��˵����Һ�����Ի����ԡ�
[�����뷴˼]
������Һ�ʼ��ԣ�����Һ��ʹ��ʼ��Ե�������OH-��
���OH-��
�ڷ���һ��þ�������ᷴӦ����������ȡ��������þ�����������ݲ�����������ԣ�������������������ԡ�
������ݲ�����
��������ȡ��������һö������������������۲쵽������ʧ����Һ��ɻ�ɫ������Ϊ�������������Ӧ�����Ȼ�����ˮ������ȡ��Һ�����ԣ���Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+6HCl�T2FeCl3+3H2O��

 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�