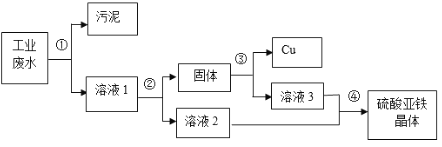
��Ŀ����
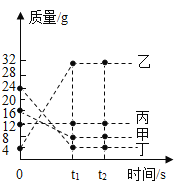
����Ŀ����ѧ��ȤС���ͬѧ��̽�����ӵ�����ʱ�������ͼ��ʾ��ʵ�飬����������ǵ�̽������ش��й����⣺�ձ�A��ʢ��10mLŨ��ˮ�����ձ�B��C�зֱ�ʢ��20mL����ˮ�����ֱ�μ�3�η�̪��Һ������ˮ�У��õ���ɫ��Һ����ֻ���ձ���A��B���ձ�����һ�𣬼����Ӻ۲�����
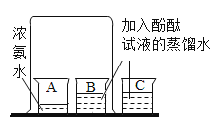
��1��ʵ���й۲쵽��������_____�������÷��ӵ�֪ʶ���ͳ��ָ������ԭ��_____��
��2���ɸ�ʵ���֪��ˮ���е������ǣ�_____��
��3��ʵ�����ձ�C��������_____��
��4����װ����һ�����ԵIJ���֮����_____����ĸĽ�������_____��
���𰸡�B�ձ��е���Һ��ɫ��ɺ�ɫ��AC�ձ���û�й۲쵽���Ե����� A�ձ��еİ������˶���B�ձ�������ˮ�ʼ��ԣ�ʹ��̪��Һ��� ��ˮ���лӷ��ԣ�ˮ��Һ�Լ��� ���� ʵ��װ���ܷ��Բ�����ݳ��������У���Ⱦ���� ��ʵ��װ�øij��ܷ���ϵ
��������
��1��ʵ���п��Թ۲쵽B�ձ��е���Һ��ɫ��ɺ�ɫ��AC�ձ���û�й۲쵽���Ե������ָ������ԭ����A�ձ��еİ������˶���B�ձ�������ˮ�ʼ��ԣ�ʹ��̪��Һ��죬���B�ձ��е���Һ��ɫ��ɺ�ɫ��AC�ձ���û�й۲쵽���Ե�����A�ձ��еİ������˶���B�ձ�������ˮ�ʼ��ԣ�ʹ��̪��Һ��졣
��2���ɸ�ʵ���֪��ˮ���лӷ��ԣ�ˮ��Һ�Լ��Ե����ʣ������ˮ���лӷ��ԣ�ˮ��Һ�Լ��ԡ�
��3��ʵ�����ձ�C��������Ϊ�˶��գ�������ա�
��4����װ����һ�����ԵIJ���֮����ʵ��װ���ܷ��Բ�����ݳ��������У���Ⱦ���������Խ�ʵ��װ�øij��ܷ���ϵ�����ʵ��װ���ܷ��Բ�����ݳ��������У���Ⱦ��������ʵ��װ�øij��ܷ���ϵ��

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�����Ŀ���±���Ca(OH)2��NaOH���ܽ�����ݡ���ش��������⣺
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��/g | Ca(OH)2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 |
NaOH | 31 | 91 | 111 | 129 | 313 | 336 | |
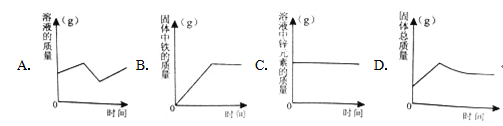
��1�������ϱ����ݣ�����Ca(OH)2��NaOH���ܽ�����ߣ���ͼ���ܱ�ʾNaOH�ܽ�����ߵ���__________������A������B������
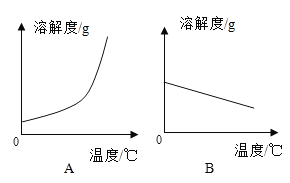
��2��Ҫ���һƿ�ӽ����͵�Ca(OH)2��Һ��ɱ�����Һ���ɲ�ȡ��ʩ��__________������ţ���
������ˮ���������¶ȣ��۽����¶ȣ��ܼ���ˮ���ݼ����������ơ�
��3������20��ʱCa(OH)2�ı�����Һ������Һ���������м���һ����CaO��ָ�20�����õ�����Һ����Һ�����ʵ����������Ĺ�ϵΪ��________�ң�����������������������������
����Ŀ����ѧ��ȤС���ͬѧ������һƿ����������Һû������Ƥ��������ʦָ���£���չ������̽����
���������1��������������Һ�Ƿ�������أ�
��ʵ��̽��1��
ʵ����� | ʵ������ | ʵ����� |
ȡ��������Һ���Թ��У�����Һ�еμ�ϡ���ᣬ�������� | ������ð���� | ����������Һһ�������ˡ� |
���������2��������������Һ��ȫ�����ʻ��Dz��ֱ����أ�
����������裩����1������������Һ���ֱ��ʡ�����2������������Һȫ�����ʡ�
���������ϣ���1���Ȼ�����Һ�����ԡ�
��2���Ȼ�����Һ����̼������Һ��Ӧ��CaCl2+Na2CO3=CaCO3��+2NaCl
��ʵ��̽��2��
ʵ�鲽�� | ʵ������ | ʵ����� |
��1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ���������� | ��________���ɡ� | ˵��ԭ��Һ��һ����̼���ơ� |
��2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ�� | ��Һ���ɫ�� | ˵��ԭ��Һ��һ����_________�� |
��ʵ����ۣ�������������Һ___________����������������ȫ���������ʡ�
����˼�����ۣ�
��1������������Һ¶���ڿ��������ױ��ʣ�������Ӧ�Ļ�ѧ����ʽ��_______________��
��2����������ʵ��̽��2���У�С�������������������Һ�����Ȼ�����Һ������Ϊ�÷���____����������������������������
��������Ӧ�ã�����������Һ���ױ��ʣ������ܷⱣ�档ʵ���ұ����ܷⱣ���ҩƷ���кܶ࣬������һ����_________________________________________��
����Ŀ��Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߡ��±���Ԫ�����ڱ���һ���֣������ݴ˱��ش��й����⡣
��һ���� | 1 H 1.008 | 2 He 4.003 | ||||||
�ڶ����� | 3 Li 6.941 | 4 Be 9.012 | 5 B 10.81 | 6 C 12.01 | 7 N 14.01 | 8 O 16.00 | 9 F 19.00 | 10 Ne 20.18 |
�������� | 11 Na 22.99 | 12 Mg 24.31 | 13 Al 26.98 | 14 Si 28.09 | 15 P 30.97 | 16 S 32.06 | 17 Cl 35.45 | 18 Ar 39.95 |
��1���ڶ������У��루Be��Ԫ�ص����ԭ������Ϊ_____����3�����У����ڽ���Ԫ�ص���_____����Ԫ�����ƣ���
��2����Ԫ�ص�ԭ�ӽṹʾ��ͼΪ��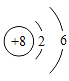 �� ��3������ijԪ������Ԫ�صĻ�ѧ�������ƣ���Ԫ�ص�ԭ�ӽṹʾ��ͼΪ��
�� ��3������ijԪ������Ԫ�صĻ�ѧ�������ƣ���Ԫ�ص�ԭ�ӽṹʾ��ͼΪ��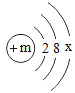 ����x=_____��m=_____��
����x=_____��m=_____��
��3������ԭ�������ֱ�Ϊ1��8��11��17������Ԫ���У�ѡ���ʵ���Ԫ��������ʣ���Щ����֮���ܷ����кͷ�Ӧ�Ļ�ѧ����ʽΪ_____����ѡ1��6��8��11�����е�Ԫ��д������Ҫ��Ļ�ѧʽһ����
�ٳ�����Ϊ��̬�ķǽ���������_____��
��������Ԫ����ɵļ�_____��
��������Ԫ����ɵ���_____��
��������ʵ������O2����ԭ�Ӹ�����Ϊ1��1������_____��