��Ŀ����
����Ŀ����ѧ��ȤС���ͬѧ������һƿ����������Һû������Ƥ��������ʦָ���£���չ������̽����
���������1��������������Һ�Ƿ�������أ�
��ʵ��̽��1��
ʵ����� | ʵ������ | ʵ����� |
ȡ��������Һ���Թ��У�����Һ�еμ�ϡ���ᣬ�������� | ������ð���� | ����������Һһ�������ˡ� |
���������2��������������Һ��ȫ�����ʻ��Dz��ֱ����أ�
����������裩����1������������Һ���ֱ��ʡ�����2������������Һȫ�����ʡ�
���������ϣ���1���Ȼ�����Һ�����ԡ�
��2���Ȼ�����Һ����̼������Һ��Ӧ��CaCl2+Na2CO3=CaCO3��+2NaCl
��ʵ��̽��2��
ʵ�鲽�� | ʵ������ | ʵ����� |
��1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ���������� | ��________���ɡ� | ˵��ԭ��Һ��һ����̼���ơ� |
��2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ�� | ��Һ���ɫ�� | ˵��ԭ��Һ��һ����_________�� |
��ʵ����ۣ�������������Һ___________����������������ȫ���������ʡ�
����˼�����ۣ�
��1������������Һ¶���ڿ��������ױ��ʣ�������Ӧ�Ļ�ѧ����ʽ��_______________��
��2����������ʵ��̽��2���У�С�������������������Һ�����Ȼ�����Һ������Ϊ�÷���____����������������������������
��������Ӧ�ã�����������Һ���ױ��ʣ������ܷⱣ�档ʵ���ұ����ܷⱣ���ҩƷ���кܶ࣬������һ����_________________________________________��
���𰸡���ɫ���� �������� ���� 2NaOH+CO2=Na2CO3+H2O ������ Ũ������лӷ���
��������
ʵ��̽��2��
��1��̼������Һ���Ȼ�����Һ��Ӧ����̼��ư�ɫ������ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ�����������ɰ�ɫ�������ɣ�˵��ԭ��Һ��һ����̼���ƣ�
��2�����裨1���м����������Ȼ�����Һ��̼��������ȫ��Ӧ��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ����Һ���ɫ��˵��ԭ��Һ��һ�����������ƣ��Ӷ��ó�[ʵ�����]��������������Һ���ֱ��ʡ�
��˼�����ۣ�
��1������������Һ¶���ڿ����У��������̼��Ӧ����̼���ƺ�ˮ��������Ӧ�Ļ�ѧ����ʽ�ǣ�![]() ��
��
��2��������ʵ��̽��2�У�С�������������������Һ�����Ȼ�����Һ����������������Һ�����Ȼ�����Һ�Dz����еģ�������ȷ�����裨2����ʵ����ۣ��ò����������Ʋ��ֱ��ʵĽ��ۣ�
��3��ʵ���ұ����ܷⱣ���ҩƷ����Ũ���ᣬŨ����ӷ��ԡ�

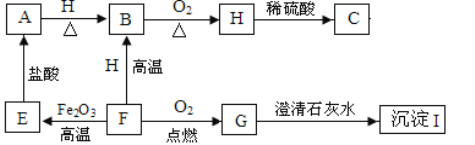
����Ŀ�����A��B��������ѡһ����������������𣬰�A�Ʒ֡�
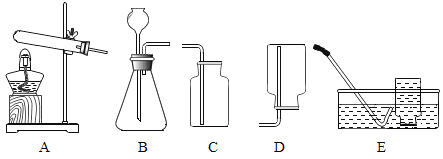
A | B |
��1��ʵ�����ø��������ȡ�����Ļ�ѧ����ʽ��________�� ��2����װ��A��E��ȡ������ѡ��װ��E�ռ�������ԭ����________�� | ��1��ʵ������ȡ������̼�Ļ�ѧ����ʽ��________ ��2����װ��B��C��ȡ������̼�����������̼���ռ����IJ�����________�� |