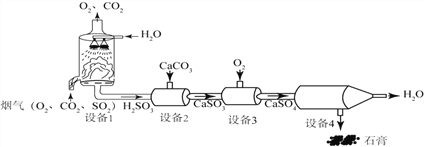
ЬтФПФкШн
ЁОЬтФПЁПЙ§бѕЛЏИЦ(CaO2)ЮЊАзЩЋЛђЕЛЦЩЋНсОЇЗлФЉЃЌЪЧвЛжжгУЭОЙуЗКЕФгХСМЙЉбѕМСЃЌПЩгУгкгуРрбјжГЁЂХЉзїЮядиХрЁЂЮлЫЎДІРэЕШЖрЗНУцЁЃЙЄвЕЩЯПЩгУДѓРэЪЏзїдСЯжЦБИЙ§бѕЛЏИЦЃЌФГаЃПЮЭтЛюЖЏаЁзщЭЌбЇФЃФтЙЄвЕЙ§ГЬжЦБИЙ§бѕЛЏИЦбљЦЗВЂВтСПЦфДПЖШЁЃ
[ВщдФзЪСЯ]ЂйЙ§бѕЛЏИЦЙЬЬхФбШмгкЫЎЃЌМгШШжС315ЁцЪБПЊЪМЗжНтЃЌЭъШЋЗжНтЕФЮТЖШЮЊ400ЁЋ425ЁцЁЃ
ЂкГЃЮТЯТЙ§бѕЛЏИЦИЩдяЧвКмЮШЖЈЁЃЙ§бѕЛЏИЦФмШмгкЯЁЫсЩњГЩЙ§бѕЛЏЧтЃЌЙ§бѕЛЏИЦдкЪЊПеЦјЛђЫЎжаЛсж№НЅЛКТ§ЕиЗжНтЃЌГЄЪБМфЗХГібѕЦјЁЃ
[ЪЕбщвЛ]ЬсДПЬМЫсИЦ
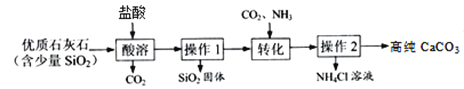
вбжЊЃКЁАзЊЛЏЁБВНжшжаЕФЗДгІЗНГЬЪНЮЊЃКCaCl2 + NH3 + CO2 + H2O ЃН CaCO3Ё§ + 2NH4Cl
(1)ЫсШмЙ§ГЬжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЪЧ___________________________________ЁЃ
(2)Й§ГЬжаЕФЩњГЩЮяПЩжБНггІгУгкСїГЬЕФЪЧ_______________ЁЃ
[ЪЕбщЖў]Й§бѕЛЏИЦЕФжЦБИЃК
вбжЊЃКЂйCaCl2 + H2O2 + 2NH3ЁЄH2O + 6H2OЃНCaO2ЁЄ8H2O + 2NH4ClЃЛ
ЂкCaO2ЁЄ8H2OВЛШмгкЫЎЃЌдк0ЁцЪБЮШЖЈЃЌМгШШжС130ЁцЪБж№НЅБфЮЊЮоЫЎCaO2ЁЃ

(3)ЮЊПижЦГСЕэЕФЮТЖШЮЊ0ЁцзѓгвЃЌдкЪЕбщЪввЫВЩШЁЕФЗНЗЈЮЊ_____________________ЁЃ
(4)ВйзїAдкЪЕбщЪвГЃгУЕФВНжшга_____________ЁЂНЕЮТНсОЇЁЂЙ§ТЫЁЂЯДЕгЁЂИЩдяЃЌДЫВйзїжаЯДЕгОЇЬхЪБВЛПЩбЁгУЕФЯДЕгМСЮЊ____________(гУађКХЬюПе)ЁЃ
aЃЎБљЫЎ bЃЎ40ЁцШШЫЎ cЃЎБЅКЭNH4ClШмвК
(5)зюКѓКцИЩЕУЕНЙ§бѕЛЏИЦЪБЃЌЪЪвЫЕФЮТЖШЗЖЮЇЮЊ_____________ЁЃ
(6)вбжЊВњЦЗЕФВњТЪ=![]() ЃЌдђДЫжЦБИСїГЬжаCaO2ЕФВњТЪЮЊ__________ЁЃ(аДГіМЦЫуЙ§ГЬЃЌНсЙћБЃСєвЛЮЛаЁЪ§ЃЌ2Зж)ЁЃ
ЃЌдђДЫжЦБИСїГЬжаCaO2ЕФВњТЪЮЊ__________ЁЃ(аДГіМЦЫуЙ§ГЬЃЌНсЙћБЃСєвЛЮЛаЁЪ§ЃЌ2Зж)ЁЃ
(7)ЪЕбщЪвЯжгаШмжЪжЪСПЗжЪ§ЮЊ37%ЁЂУмЖШЪЧ1.18gЁЄmL-1ЕФХЈбЮЫсЃЌдђХфжЦ12%ЕФЯЁбЮЫс100gЃЌашвЊЫЎ____________mL(ЫЎЕФУмЖШЪЧ1gЁЄmL-1ЃЌМЦЫуНсЙћБЃСєвЛЮЛаЁЪ§)ЁЃ
[ЪЕбщШ§]Й§бѕЛЏИЦКЌСПЕФВтЖЈ
ИУЛюЖЏаЁзщЩшМЦЯТСазАжУЃЌЭЈЙ§ВтЖЈЩњГЩO2ЕФЬхЛ§ЃЌМЦЫубљЦЗжаCaO2ЕФжЪСПЗжЪ§ЁЃ
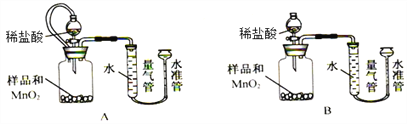
вбжЊЃКa.CaO2 + 2HCl = CaCl2 + H2O2 b.дгжЪВЛгыбЮЫсЗДгІЩњГЩЦјЬх
(8)ЪЕбщжаMnO2ЕФзїгУЪЧ____________________ЁЃ
(9)ЯрЭЌЬѕМўЯТЃЌ_______________ФмЪЙВтЖЈНсЙћИќзМШЗ(ЬюЁАзАжУAЁБЛђЁАзАжУBЁБ)ЃЌ МьВщИУзАжУЦјУмадЕФВйзїЪЧ____________________________________ЁЃСэвЛЬззАжУВтЖЈНсЙћВЛзМШЗЕФжївЊдвђЪЧ________________________________ЁЃ
[ЙлВьЩњЛю]
(10)ЮЊЗРжЙЙ§бѕЛЏИЦдкПеЦјжаБфжЪЃЌгІ____________БЃДцЁЃ
ЁОД№АИЁП ![]() CO2 НЋЗДгІШнЦїжУгкБљЫЎжа(ЛђБљЫЎдЁ) МгШШХЈЫѕ(ЛђеєЗЂХЈЫѕ) b 130ЁцЁЋ315Ёц 97.2% 67.6 ДпЛЏзїгУ зАжУA ЯђЫЎзМЙмжаМгЫЎЃЌвЛЖЮЪБМфКѓгыСПЦјЙмгаЮШЖЈЕФИпЖШВюЃЌдђЫЕУїЦјУмадСМКУЁЃ МгШыЕФЯЁбЮЫсвВеМСЫвЛЖЈЕФЬхЛ§ЃЌЪЙНсЙћЦЋДѓ УмЗт
CO2 НЋЗДгІШнЦїжУгкБљЫЎжа(ЛђБљЫЎдЁ) МгШШХЈЫѕ(ЛђеєЗЂХЈЫѕ) b 130ЁцЁЋ315Ёц 97.2% 67.6 ДпЛЏзїгУ зАжУA ЯђЫЎзМЙмжаМгЫЎЃЌвЛЖЮЪБМфКѓгыСПЦјЙмгаЮШЖЈЕФИпЖШВюЃЌдђЫЕУїЦјУмадСМКУЁЃ МгШыЕФЯЁбЮЫсвВеМСЫвЛЖЈЕФЬхЛ§ЃЌЪЙНсЙћЦЋДѓ УмЗт
ЁОНтЮіЁП(1) (2)ИљОнЬтжааХЯЂЗжЮіНтД№ЃЛ(3) вЊПижЦдк0ЁцзѓгвЃЌПЩвддкБљЫЎЛьКЯЮяжаПижЦЃЛ(4)ИљОнгЩШмвКЕУЕНОЇЬхЕФЗНЗЈЗжЮіНтД№ЃЛИљОнОЁПЩФмЖрЕФдіМгВњЦЗжЪСПЗжЮіНтД№ЃЛ(5)ИљОнЬтжааХЯЂЗжЮіНтД№ЃЛ(6) (7)ИљОнМЦЫуНтД№ЃЛ(8)ИљОнЖўбѕЛЏУЬВЛгыЯЁбЮЫсЗДгІЃЌЕЋФмИФБфЙ§бѕЛЏЧтЕФЗжНтЫйТЪНтД№ЃЛ(9)ИљОнзАжУЬиЕуЗжЮіНтД№ЃЛ(10)ИљОнЮЊЗРжЙЙ§бѕЛЏИЦдкПеЦјжаБфжЪЃЌгІИєОјПеЦјНтД№ЁЃ(1)гЩЬтжааХЯЂПЩжЊЃЌЫсШмЙ§ГЬЪЧЯЁбЮЫсгыЪЏЛвЪЏЃЈжївЊГЩЗжЬМЫсИЦЃЉЗДгІЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊCaCO3+2HCl=CaCl2+H2O+CO2ЁќЃЛ(2) гЩЬтжааХЯЂПЩжЊЃЌCO2КЭNH3гУгкзЊЛЏВНжшЁЃЖјCO2ЪЧЫсШмВНжшЕФВњЮяЃЌЙЪЙ§ГЬжаЕФЩњГЩЮяПЩжБНггІгУгкСїГЬЕФЪЧCO2ЃЛ(3) ЮЊПижЦГСЕэЕФЮТЖШЮЊ0ЁцзѓгвЃЌдкЪЕбщЪввЫВЩШЁЕФЗНЗЈЮЊНЋЗДгІШнЦїжУгкБљЫЎжа(ЛђБљЫЎдЁ)ЃЛ(4) ВйзїAдкЪЕбщЪвГЃгУЕФВНжшгаМгШШХЈЫѕ(ЛђеєЗЂХЈЫѕ)ЁЂНЕЮТНсОЇЁЂЙ§ТЫЁЂЯДЕгЁЂИЩдяЃЛвЊОЁПЩФмЖрЕФдіМгВњЦЗжЪСПЃЌВЛФмгУШШЫЎЯДЕгЃЌЙЪбЁbЃЛ(5) CaO2ЁЄ8H2OМгШШжС130ЁцЪБж№НЅБфЮЊЮоЫЎCaO2ЃЌМгШШжС315ЁцЪБПЊЪМЗжНтЁЃЙЪзюКѓКцИЩЕУЕНЙ§бѕЛЏИЦЪБЃЌЪЪвЫЕФЮТЖШЗЖЮЇЮЊ130ЁцЁЋ315ЁцЃЛ
(6) ЩшЩњГЩТШЛЏИЦЕФжЪСПЮЊAЁЃ
CaCO3+2HCl=CaCl2+H2O+CO2Ёќ
100 111
20g A
![]()
A=22.2g
ЩшЩњГЩCaO2ЁЄ8H2OЕФжЪСПЮЊBЁЃ
CaCl2+H2O2+2NH3ЁЄH2O+6H2OЃНCaO2ЁЄ8H2O + 2NH4Cl
111 216
22.2g B
![]()
B=43.2g
43.2g CaO2ЁЄ8H2OжаCaO2ЕФжЪСПЮЊ43.2gЁС![]() ЁС100ЃЅ=14.4gЁЃДЫжЦБИСїГЬжаCaO2ЕФВњТЪЮЊ
ЁС100ЃЅ=14.4gЁЃДЫжЦБИСїГЬжаCaO2ЕФВњТЪЮЊ![]() ЁС100ЃЅ=97.2ЃЅЃЛ(7) ЩшашвЊХЈбЮЫсЕФжЪСПЮЊxЁЃ37%x=100gЁС12%ЃЌx=32.4gЃЛашвЊЫЎЕФжЪСП=100g-32.4g =67.6gЃЌЫЎЕФЬхЛ§=67.6gЁТ1gЁЄmL-1=67.6gЁЄmL-1ЃЛ(8) ЪЕбщжаMnO2ЕФзїгУЪЧИФБфЙ§бѕЛЏЧтЕФЗжНтЫйТЪЃЌЦ№ДпЛЏзїгУЃЛ(9) ЯрЭЌЬѕМўЯТЃЌзАжУAФмЙЛБЃжЄЬхЯЕбЙЧПВЛЪмМгШыбЮЫсЬхЛ§ЕФгАЯьЃЌЫљвдМгШыбЮЫсЪБВЛЛсгАЯьЩњГЩЕФбѕЦјЕФЬхЛ§ВтЖЈЃЌЖјзАжУBбЮЫсЕФМгШыЛсЕМжТСПЦјЭВвКУцУїЯдЯТНЕЃЌЛсгАЯьНсЙћЃЌЙЪзАжУAФмЪЙВтЖЈНсЙћИќзМШЗЃЛМьВщAзАжУЦјУмадЕФВйзїЗНЗЈЪЧЯђЫЎзМЙмжаМгЫЎЃЌвЛЖЮЪБМфКѓгыСПЦјЙмгаЮШЖЈЕФИпЖШВюЃЌдђЫЕУїЦјУмадСМКУЃЛ(10)ЮЊЗРжЙЙ§бѕЛЏИЦдкПеЦјжаБфжЪЃЌгІУмЗтБЃДцЁЃ
ЁС100ЃЅ=97.2ЃЅЃЛ(7) ЩшашвЊХЈбЮЫсЕФжЪСПЮЊxЁЃ37%x=100gЁС12%ЃЌx=32.4gЃЛашвЊЫЎЕФжЪСП=100g-32.4g =67.6gЃЌЫЎЕФЬхЛ§=67.6gЁТ1gЁЄmL-1=67.6gЁЄmL-1ЃЛ(8) ЪЕбщжаMnO2ЕФзїгУЪЧИФБфЙ§бѕЛЏЧтЕФЗжНтЫйТЪЃЌЦ№ДпЛЏзїгУЃЛ(9) ЯрЭЌЬѕМўЯТЃЌзАжУAФмЙЛБЃжЄЬхЯЕбЙЧПВЛЪмМгШыбЮЫсЬхЛ§ЕФгАЯьЃЌЫљвдМгШыбЮЫсЪБВЛЛсгАЯьЩњГЩЕФбѕЦјЕФЬхЛ§ВтЖЈЃЌЖјзАжУBбЮЫсЕФМгШыЛсЕМжТСПЦјЭВвКУцУїЯдЯТНЕЃЌЛсгАЯьНсЙћЃЌЙЪзАжУAФмЪЙВтЖЈНсЙћИќзМШЗЃЛМьВщAзАжУЦјУмадЕФВйзїЗНЗЈЪЧЯђЫЎзМЙмжаМгЫЎЃЌвЛЖЮЪБМфКѓгыСПЦјЙмгаЮШЖЈЕФИпЖШВюЃЌдђЫЕУїЦјУмадСМКУЃЛ(10)ЮЊЗРжЙЙ§бѕЛЏИЦдкПеЦјжаБфжЪЃЌгІУмЗтБЃДцЁЃ

 53ЬьЬьСЗЯЕСаД№АИ
53ЬьЬьСЗЯЕСаД№АИЁОЬтФПЁПФГжжПѓЪЏгЩбѕЛЏУОЁЂбѕЛЏЬњЁЂбѕЛЏЭКЭЖўбѕЛЏЙшзщГЩЃЌгУЫќжЦБИЧтбѕЛЏУОЕФСїГЬЪОвтЭМШчЭМЫљЪО(вбжЊ:ЖўбѕЛЏЙшВЛШмгкЫЎвВВЛгыЯЁбЮЫсЗДгІ)ЁЃ
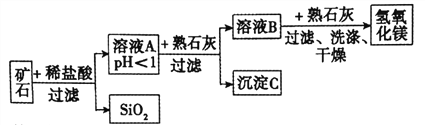
ЧыЛиД№ЯТСаЮЪЬтЁЃ
(1)МгЯЁбЮЫсжЎЧАвЊНЋПѓЪЏЗлЫщЕФФПЕФЪЧ_____________ЁЃ
(2)ШмвКAжаГ§СЫMg2+ЭтЃЌЛЙКЌгаЕФН№ЪєбєРызгЪЧ______________;(аДРызгЗћКХ)
аДГіПѓЪЏжаЕФШЮвтвЛжжН№ЪєбѕЛЏЮягыЯЁбЮЫсЗДгІЕФЛЏбЇЗНГЬЪН:______________(жЛаДвЛИі)ЁЃ
(3)дкШмвКAжаМгШыЪьЪЏЛвЕїНкШмвКЕФpHЃЌПЩвдЪЙШмвКжаЕФН№ЪєбєРызгж№ВНзЊЛЏЮЊГСЕэЁЃИУЪЕбщЬѕМўЯТЃЌЪЙН№ЪєбєРызгГСЕэЕФЯрЙиpHЪ§ОнМћЯТБэЁЃЮЊБЃжЄВњЦЗДПЖШЁЂМѕЩйВњЦЗЫ№ЪЇЃЌВЂБугкВйзїЃЌЫљЕУШмвКBЕФpHЕФШЁжЕЗЖЮЇЮЊ______________ЁЃ(гУВЛЕШЪНБэЪО)
ЧтбѕЛЏЮя | Fe(OH)3 | Cu(OH)2 | Mg(OH)2 |
ПЊЪМГСЕэЕФpH | 1.5 | 4.2 | 8.6 |
ЭъШЋГСЕэЕФpH | 3.2 | 6.7 | 11.1 |
(4)аДГіШмвКBжаМгШыЪьЪЏЛвЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪН:___________________________ЁЃ