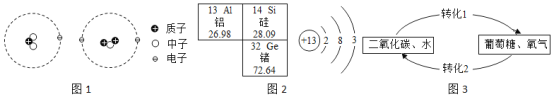
��Ŀ����
����Ŀ��ʵ��һ����ͼI�ǽ̲�ʵ��,ʵ���пɹ۲쵽��������_______________��
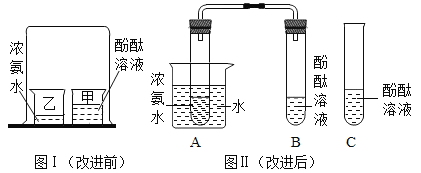
ʵ�����ʵ����ͬѧ���ŵ��˴̼�����ζ�����Ǵ�Ҷ�ʵ������˸Ľ�,װ����ͼ����ʾ��
ʵ�������
a��B��C��E��֧�Թ��зֱ����5 mL����ˮ��������1~2����ɫ��̪��Һ,���۲���Һ��ɫ��
b��A��D�Թ��зֱ����3 mLŨ��ˮ�������ô���Ƥ���ĵ��ܰ�ʵ��ͼ�����Ӻã�����D�Թܷ�����ʢ����ˮ���ձ���,�۲켸���ӡ�
�������ۣ�
��1��E�Թ��з��з�̪��Һ��Ŀ����_______________��
��2�����в���bʱ�۲쵽��������_______________��
��3���ɴ˿��Եõ���ʵ����ۣ���______________����________________��
��4���ԱȸĽ�ǰ��ʵ��,�Ľ����ʵ����ŵ���______________��
��5���������ʵ��,ʵ���ұ���Ũ��ˮ��ҩƷʱ��Ӧ��ע�����__________________��
���𰸡����ձ��б�죬���ձ��������Ա仯 ��B��C�Թܽ��ж��� C�Թ�����ҺѸ�ٱ�죬B�Թ�����Һ��һ���� �����Dz����˶��� �¶�Խ�߷��ӵ��˶�Խ���� �����Ų����̼�����ζ����С������Ⱦ Ӧ��Ũ��ˮ�ܷⱣ����������
��������
һ��Ũ��ˮ���лӷ��ԣ��ӷ�����������̪��Һʹ���죬�ʿ��Թ۲쵽���ձ��б�죬���ձ��������Ա仯��
��1��E�Թ��з��з�̪��Һ��Ŀ������B��C�Թܽ��ж��գ�
��2�����в���bʱ�۲쵽��������C�Թ��¶ȸߣ�������ҺѸ�ٱ�죬B�Թ����¶ȵͣ���Һ��һ���죻
��3��B��C�Թ��е���Һ����죬˵���������ӽ���B��C�����̪��Һ��Ӧ��ʹ���죬˵�������Dz����˶��ģ���C�Թܱ�B�Թ��ȱ�죬˵���¶�Խ�߷��ӵ��˶�Խ���ң�
��4���ŵ��ǻ����Ų����̼�����ζ����С������Ⱦ��
��5��Ũ��ˮ�ӷ������ʵ���ұ���ʱӦ�ܷⱣ������������

����Ŀ�����������ʵ�飬�ش����⡣
ijͬѧ�������������ϣ��ڰ�ȫ����������ʦָ���µ�ȼ��ʵ������
V��H2��/V�������� | 1��99 | 3��97 | 4��96 | 50��50 | 70��30 | 75��25 |
��� | ��ը | ��ը | ը | ը | ը | ��ը |
��1����ʵ��˵�������ı�ը����Χ����Ϊ_____��_____֮�䣨�ðٷ�����ʾ��
��2�����ڸ�ʵ�鱬ը��Σ���ԣ�˵����ȼ����ǰ�����鴿���鴿�IJ���Ϊ_____��
��3����ʵ��֤��ˮ����_____��ɵġ�
����Ŀ��ij��ѧ��ȤС��Ϊ�˲ⶨ��п��п����������,��10g��п�з�5�ι�����50.0gϡ���ᣨ���ʲ������ᷴӦ��,�����������±���ʾ������㣺
���������������/g | 10 | 20 | 40 | 50 |
��������/g | 0.1 | 0.2 | m | 0.3 |
��1��������40g����ʱ,��Ӧ����������m��___________g��
��2����������_______________________��
����Ŀ��ij̼�����Ʒ�к��в������ᷴӦ�����ʡ�ijͬѧȡ12g����Ʒ���ձ��У������ձ��н�100gϡ�������μ�����Ʒ�У���ַ�Ӧʹ����ȫ���ݳ��Ƶ��ձ�����ʢ���ʵ����������������ձ��������������±���ʾ��
ʵ����� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
����ϡ���������/g | 20 | 20 | 20 | 20 | 20 |
�ձ�����ʢ���ʵ�������/g | 30.90 | 49.80 | m | 87.60 | 107.05 |
����գ�
��1��̼��ƺ����ᷴӦ�Ļ�ѧ����ʽΪ_____��
��2��m��ֵΪ_____g����
��3��12gʯ��ʯ��Ʒ��̼��Ƶ�������������_____��
��4����Ӧ��ȫ����Һ���Ȼ��Ƶ�����_____��д������̣�������λС������
����Ŀ����ҵ������Ҫ�ɷ���Fe2O3��������������FeO��Fe3O4
���������ϡ�(1)���ᾧ��(H2C2O43H2O)��Ũ�������������ȷֽ⣬��ѧ����ʽΪ��H2C2O43H2O ![]() CO2��+CO��+H2O
CO2��+CO��+H2O
(2)��ʯ���ǹ���NaOH��CaO�Ļ���������ˮ�����Ͷ�����̼��
(3)���ij�������������������������
���������� | FeO | Fe2O3 | Fe3O4 |
������������ | 77.8% | 70.0% | 72.4% |
���������ۡ�Ϊ�˲ⶨ��������������������С����������ʵ�顣(װ������������)
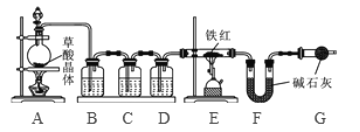
(1)��ʵ��Ϊ�˱�֤����E�е������Ǵ����������CO����B��C��D�е��Լ�������________(����ĸ���)
a��Ũ���� b�������ʯ��ˮ c����������Һ
(2)Cװ�õ������� __________________________��
(3)д��Eװ������������Ӧ��һ����ѧ����ʽ�� _____________________��
(4)��ȡ������Ʒ10.0g,������װ�ý���ʵ�飬�ⶨ��������������������
����E�г�ַ�Ӧ��õ����۵�����Ϊmg���� ____ < m < ______��
����ʵ��ǰ��Ƶ�Fװ�É���7.7g������������������������� _________��
��ʵ�鷴˼�� (1)���ȱ��Gװ��(��������������)��������Ʒ���������������� ________(ѡ����ƫС������������ƫ����)��
(2)��ʵ��װ�õ�һ������ȱ���� ___________________��