��Ŀ����
��Ҫ��д����ѧ����ʽ���ش��й����⣺
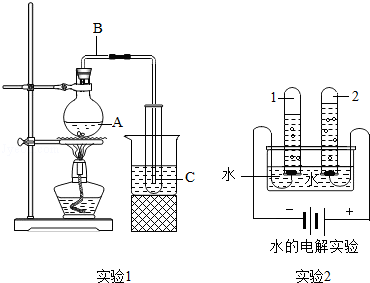
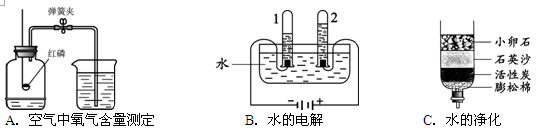
��1��ˮ��ͨ��ʱ������⣺��_ ________����
�ڸ�ʵ���У��ɿ��������븺������������������_________������ʵ��ó��Ľ�����ˮ����____ _____��ɡ�
��2��ϸ��˿�ڴ�������ȼ�գ���__ _______����
��ʵ����Ҫ����ƿ��Ԥ�ȷ�������ˮ����ϸɳ��ԭ������___ __ ____ ����
��3����ͼ��ͼ�к���ȼ�յĻ�ѧ����ʽΪ��_______ __ ����
��ֹˮ�к�����������_______ __��˵��������������Լռ_ ___������ͨ��ʵ��ó�������۵Ļ�ѧ����__ __������ţ���
| A�������� | B�������� | C�������� | D����ķ�� |
��1��2H2O 2H2��+ O2 �� 1��2 ��Ԫ�غ���Ԫ��
2H2��+ O2 �� 1��2 ��Ԫ�غ���Ԫ��
��2��3Fe + 2O2 Fe3O4 ��ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը��
Fe3O4 ��ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը��
��3��4P + 5O2 2P2O5 ˮ����ƿ�У���Լռƿ�������������1/5 1/5 A
2P2O5 ˮ����ƿ�У���Լռƿ�������������1/5 1/5 A
���������������1�����ˮʵ�飬���Դ������������������ʹ�����ǵ�ľ����ȼ�������������Դ��������������������ȼ�գ���������ɫ�Ļ��桪�����������Է���ʽ��2H2O 2H2��+ O2 �������ˮ�ھ����������⣬�����һ�����������غ㶨�ɣ�Ԫ�ص������ڷ�Ӧǰ�䣬���Խ����ǣ�ˮ����Ԫ�غ���Ԫ�����
2H2��+ O2 �������ˮ�ھ����������⣬�����һ�����������غ㶨�ɣ�Ԫ�ص������ڷ�Ӧǰ�䣬���Խ����ǣ�ˮ����Ԫ�غ���Ԫ�����
��2����˿ȼ�յķ���ʽ��3Fe + 2O2 Fe3O4��Ϊ�˷�ֹ��ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը�ѣ�Ҫ����ƿ��Ԥ�ȷ�������ˮ����ϸɳ
Fe3O4��Ϊ�˷�ֹ��ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը�ѣ�Ҫ����ƿ��Ԥ�ȷ�������ˮ����ϸɳ
��3������ȼ�յķ���ʽ��4P + 5O2 2P2O5�����ں���ȼ��������ƿ�ڵ�������ʹ��ƿ�ڵ����������٣�ѹǿ���٣�����ˮ����ƿ�У���Լռƿ�������������1/5������ˮ����ƿ�У���Լռƿ�������������1/5��˵�������������ĺ�����Լ��1/5������ͨ��ʵ��ó�������۵Ļ�ѧ���ǣ�����������ѡA
2P2O5�����ں���ȼ��������ƿ�ڵ�������ʹ��ƿ�ڵ����������٣�ѹǿ���٣�����ˮ����ƿ�У���Լռƿ�������������1/5������ˮ����ƿ�У���Լռƿ�������������1/5��˵�������������ĺ�����Լ��1/5������ͨ��ʵ��ó�������۵Ļ�ѧ���ǣ�����������ѡA
���㣺ˮ�ĵ��ʵ�飬��˿ȼ�գ����������������IJⶨ

 ��У����ϵ�д�
��У����ϵ�д���Ϣ����ʮ�������������ϵ����ʲ��ϵر仯�������ijɷ�Ҳ�����˺ܴ�ı仯���±���ԭʼ������Ŀǰ��������Ҫ�ɷ֣�
| Ŀǰ��������Ҫ�ɷ� | N2��O2��CO2��ˮ��������������� |
| ԭʼ��������Ҫ�ɷ� | CH4��NH3��CO��CO2�� |
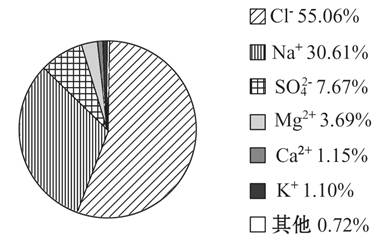
��Ϣ����ͼ�ֱ��Ǻ�ˮ�������в���Ԫ�ص�����������

���������ṩ����Ϣ��������ѧ�����й�֪ʶ�ش��������⣺
��1���ں�ˮ�У�����Ԫ�غ�����ߵ�����Ԫ�ء� �ҹ��ຣ���Ǹ��ں������ˮ�Dz����������У���ˮ�뺣ˮ����Ҳ���̵ģ��������϶����ĺ�ˮ�ǵ��ġ���������ˮ���ܽ�Ĺ��ɺ�ˮ����Ȼѭ���ĽǶȿ�����ˮ����Ԫ�صĺ����Ƚϸߵ�ԭ����______________________________________________��
��2��ˮ����Ԫ�ص����������Ⱥ�ˮ����Ԫ�ص��������������С����________��
������̼Ԫ�ص���Ժ����϶��ԭ����_______________________________________��
��3��ԭʼ������ָ��ɫֲ�������ǰ�Ĵ�����������ִ����˻������Ķ�ֲ�����ԭʼ�����У�����Ϊ�����ܷ�������ȥ��________��ԭ����ʲô_________________________________________________��
��4����ɫֲ������Ժ�ԭʼ�����е�CO2���١�ͬʱO2�����ӣ�ԭ����________________________________________________��
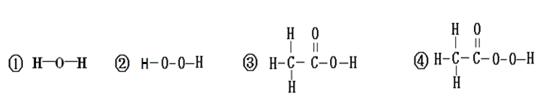


 Cu+H2O��
Cu+H2O��