��Ŀ����
��6�֣���ѧ�о����ʵ���ɡ��ṹ�����ʡ��仯֮��Ĺ�ϵ��
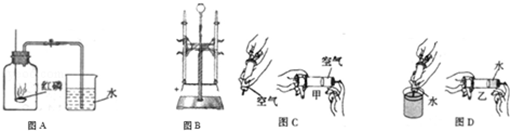
��1����ѧ��ͨ���о�ˮ�ı仯��ʶ��ˮ����ɡ���ͼΪ���ˮ�ļ���װ�ã��Թܢ��в����������� ���ɴ�ʵ��ó�ˮ���� ��ɵģ�2����ɺ������������е���ϵ������Һ�������ƵĻ�ѧ��������Ϊ���ж����� ��
��3���ṹ�������ʡ��о����ֺ��С�������������O��O���������ʾ��к�ǿ�������ԣ�������Ϊɱ�����������ݴ��Ʋ⣬���������У�������ɱ������������ (���������)��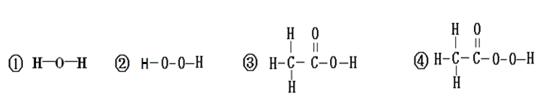
��4��ͨ��������ɺͽṹ�����ǿ���Ԥ�����ʵ�ijЩ���ʡ�����NaHSO4���ʵ������Ʋ⣬�������� ����������ţ���
����ˮ��Һ����ط����û���Ӧ���õ�������
����ˮ��Һ��ʹ��ɫʯ����Һ���
����ˮ��Һ����п��Ӧ��������
����ˮ��Һ�������ᱵ��Ӧ�������ᱵ����������ʾ�����ᱵ������ˮ��Ҳ�������ᣩ
��1������ ��Ԫ�غ���Ԫ�� ��2����Ԫ�� ��3���ڢ� ��4���ڢۢ�
���������������1�����ˮ�ļ���װ�ã��Թܢ��в������������������ɴ�ʵ��ó�ˮ������Ԫ�غ���Ԫ����ɵġ���2����ɺ������������е���ϵ������Һ�������ƵĻ�ѧ��������Ϊ���ж�������Ԫ�أ���3���ṹ�������ʡ��о����ֺ��С�������������O��O���������ʾ��к�ǿ�������ԣ�������Ϊɱ�����������ʿ�����ɱ�����������ǻ�ѧʽ�к��й����������ʢڢܣ���4��ͨ��������ɺͽṹ�����ǿ���Ԥ�����ʵ�ijЩ���ʡ�����NaHSO4���ʢ���ˮ��Һ��ʹ��ɫʯ����Һ������ˮ��Һ����п��Ӧ������������ˮ��Һ�������ᱵ��Ӧ�������ᱵ������
���㣺���ʵ���ɡ��ṹ������

 53������ϵ�д�
53������ϵ�д���7�֣�ˮ����Һ������������������ʮ����Ҫ�����á�
��1����ͼ�ǵ��ˮʵ��װ�á���ʵ������У��Թ�1������������ ��д��ˮ��ͨ�������·�Ӧ�Ļ�ѧ����ʽ ��
��2����Դˮ����������ˮ�Ĺ������������ʯ�ң���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ__ __��
��3��20��ʱ���Ȼ��Ƶ��ܽ��Ϊ36g����20��ʱ�Ȼ��Ʊ�����Һ�����ʺ��ܼ���������Ϊ ��
��4��Ϊ�˽���ũҵѡ�֣��ֽ�200g30%���Ȼ�����Һϡ��Ϊ10%���Ȼ�����Һ����Ҫ��ˮ������Ϊ ��
��5������ˮ��ͨ��������������ɱ������������������Ũ��ˮ������������豸�����������Ƿ�������й©��A��B��C��D��ʾ4�����ʣ�����ʾ��ͼ���±���A��B��һ�������·�Ӧ����C��D��
| ���� | A | B | C | D |  |
| ��ѧʽ | NH3 | Cl2 | N2 | | |
| ��ʾ��ͼ |  |  |  |  |
��17 g A�μӷ�Ӧ��������C������Ϊ g��
ˮ�ڻ�ѧʵ���е����ò��ɺ��ӡ���ͼ��ʾ���ĸ�ʵ���зֱ��õ�ˮ��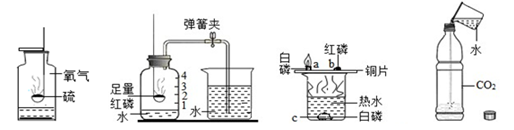
| A������������ȼ�� | B���ⶨ�������������� | C��̽��ȼ������ | D��̽��������̼���� |
��1��ʵ��A��ˮ�������� ��
��2��ʵ��B�м���ƿ��ˮ����Ҫ������ ��
��3��ʵ��C����ˮ���������ṩ������ ��
��4��ʵ��D��ˮ�������� �� ��
��10�֣���ѧ������������ء�������صĻ�ѧ֪ʶ�ش��������⣺
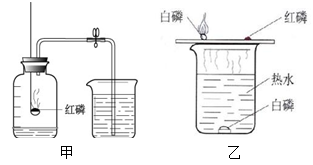
��1�����������������ཡ�������봶�ߣ���Ҫԭ���� ������������ˮ��
�ȷ�����Ӧ�����⣬д��һ��Ԥ����������ľ������� ��
��2���ٽ��п�������Ϊ�˸�С������Ӫ�����ƶ������ʳ�����£�����ը���ȡ������㡢ţ�̡�ʳ���и��������ʳ���� ��Ϊ��Ӫ�����⣬�㽨��С�������軹Ӧ���ӵ�ʳ���� ��дһ�֣���
��3����������ҹ���س���������������PM2.5��������������ġ����ס�֮һ��
��PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ����Ҳ�ƿ���ο����������Ϊ�����PM2.5���� ��
| A����У�������ѻ��������͵ط��� | B��Ϊ������У��ᳫ���������ʹ��˽�ҳ� |
| C��������չ�������� | D��������ȼ�ű��� |
2013 �������������ͻȪ��������������̶�����Ǻӵ�������Դ���齨���µ�һȪ�羰�����������ص���Ȼɽˮ���ۺ�������ʷ�Ļ�������һ�壬Ϊ 5A ��������
��1����ͻȪˮ����__________����������������
��2��ij��ѧС���ͬѧ�Ի��Ǻӵ�ˮ����������ص��о�����Ҫ�� pH ��ֽ���Բⶨ��ˮ�������ǿ�����ⶨ�ľ��巽����
��3��Ϊ�˼��������ˮ����ˮ����Ӳˮ������ˮ���м���___________�����衣
��4��������Ȫˮ֮������Ȫˮ������˵���д������ ������ţ�
| A���峺��Ȫˮ����Һ | B�������Ǿ����̶���ߵľ�ˮ���� |
| C��������еķ�������Ȫˮ��Ӳ�� | D�����˿��Գ�ȥȪˮ�еĿ��������� |