��Ŀ����
������ˮ�Ͷ�����̼������������Ҫ�����ʡ�
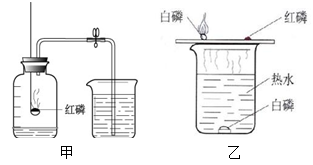
��1��A��B��C�����о�������ɺ����ʵ�ʵ�顣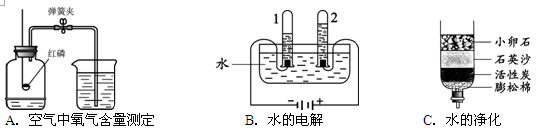
�ٹ���Aͼ��ʾʵ�飬����˵������ȷ���� ��
A��ʵ��ʱ����Ӧ���� B����ȼ����ǰ���õ��ɼмн��齺��
C������Ϩ������̴��ɼ� D�����ս���ƿ��ˮ�����ԼΪ���������
��Bͼ�Թ�1�е�����Ϊ ��Cͼ�о���ˮ�ķ����� ��������
��2����ʯ�ҽ�Ĩǽ��һ��ʱ���ǽ�ڱ���ְ���Ӳ��ԭ���� ���û�ѧ����ʽ�ش𣩡�
��3��������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ�¶�����̼�Ͱ�����NH3�����Ժϳ�����[CO(NH2)2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����̼�����Ե��ܺġ����ŷš�����ȾΪ��������ʵ���������Դ����Ч�ʺʹ��������Դ�ṹ������˵���У���ȷ���� ��
A����������ʱ����ú������ú����ʹ��
B����У��������͵��������շ��糧�����շ���
C���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ�
D������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դx
��1����C ��H2 ����
��2��Ca(OH)2+CO2=CaCO3��+H2O
��3��CO2+2NH3 H2O+CO(NH2)2
H2O+CO(NH2)2
��4��ABCD
���������������1���ٸ�ʵ���Dzⶨ����������������ʵ�顣��ʵ���ԭ�����������ʽ�������Ӧ�������������ѹ���ͣ�����ˮ��������Dz��뷴Ӧ�������������ʵ��ɹ����Ĺؼ��У�1������Ҫ������2��װ��������Ҫ���ã�3������ʱ�¶�Ҫ�������¡�����C�������ɶ���ƫ�ͣ���
��BͼΪ���ˮʵ�顣�Թ�1�е�����������Թ�Ҫ�࣬���Թ�1���Դ�ĸ������������Բ���������Ϊ�������Թ�2�ڵ�����Ϊ������
Cͼ������С��ʯ��ʯӢɳ�����ʶ�ˮ�����˹��˾��������û���̿��ˮ����������������
��2��ʯ�ҽ��ijɷ����������ƣ�����������еĶ�����̼��Ӧ����ѧ����ʽΪ��Ca(OH)2+CO2=CaCO3��+H2O
��3�������п�֪����Ӧ��Ϊ�����Ͷ�����̼������Ϊ���¸�ѹ��������Ϊ���غ�ˮ�����Է�Ӧ�Ļ�ѧ����ʽΪ��CO2+2NH3 H2O+CO(NH2)2
H2O+CO(NH2)2
��4��A����������ʱ����ú������ú����ʹ�ÿ����ӿ�ȼ���������ĽӴ�������Ӷ��ﵽ���ȼ�������ʵ����á�����Ҫ��B����У��������͵��������շ��糧�����շ��硱�ȼ�С����Щ������������Ⱦ�����γ�һ���ĵ��ܡ�����Ҫ��C���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ硱�����˶�̫���ܵ����ã������˶Ե��ܵ�������Ҫ��D������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ�������˶�̫���ܵ����á�����Ҫ��
���㣺���������ʡ���ѧ����ʽ����д����ɫ��ѧ

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�ˮ������ͨ�����������֮һ��
��1�������еġ�ˮ���кܶ��֡����С�ˮ�����ڴ�������� (����ĸ���)��
| A����ˮ | B������ˮ | C������ˮ | D����Ȫˮ |
��3����ͼΪ���ˮ��װ�ã�ͨ��һ��ʱ����Թ�2�����ռ�������Ϊ ��������Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ���� _������ϡ��ֽ⡱����Ӧ��


��4��ˮ��һ����Ҫ�Ļ���ԭ�ϡ��ȼҵͨ����ⱥ���Ȼ�����Һ�ķ�����ȡ�ռNaOH����ͬʱ������������������Cl2�����䷴Ӧ�Ļ�ѧ����ʽΪ ��