��Ŀ����
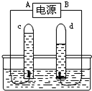
ijͬѧΪ�ⶨ�����������ĺ������������ͼ��ʾ��ʵ��װ�á���ͬѧ�ڡ������ݡ���ÿһ���İ�������һ������ֽ����ˮ��İ��ף��÷Ŵ��6V�ֵ�Ͳ���ڿ���ˮ���һ���������ݡ����İ����ϡ�
��1��һ��ʱ��ɹ۲쵽����Ҫ������ ��
��2���������ݡ���ÿһ���϶�����һС�Ű�����ֻ����ˮ���һ���������ݡ�����һ��Ű�����ȣ��ŵ��� ��
��3��д������ѧ���IJ�����ʵ������ķ�Ӧ�Ļ�ѧ����ʽ�� ��
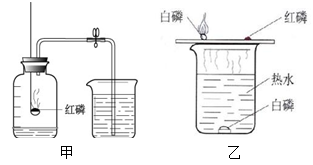
��1����������¥�ݡ�������ȼ�գ���ʼʱð���̣�ȼ�տ�ֹͣʱ�ڵ����Թܵ��ϲ����ֻ��̡����ڰ���ȼ���������Թ��ڵ�������ʹ�Թ��ڵ�ˮλ������һ��λ�ú�㶨����
��2�������ܶ�������Թ��ڵ�����
��3��4P��5O2��2P2O5
�������������(1)�����Ż��ͣ��÷Ŵ��6V�ֵ�Ͳ���ڿ���ˮ���һ���������ݡ����İ����ϣ�����������¥�ݡ�������ȼ�գ���ʼʱð���̣�ȼ�տ�ֹͣʱ�ڵ����Թܵ��ϲ����ֻ��̡��Թ��ڵ�ˮλ������һ��λ�ú�㶨����
(2) �������ݡ���ÿһ���϶�����һС�Ű�����ֻ����ˮ���һ���������ݡ�����һ��Ű�����ȣ��ŵ��Ǿ����ܶ�������Թ��ڵ�����
(3) ����ȼ�շ�Ӧ�Ļ�ѧ����ʽ��4P��5O2��2P2O5
���㣺���������������IJⶨ

 һ����������ϵ�д�
һ����������ϵ�д���7�֣�ˮ����Һ������������������ʮ����Ҫ�����á�
��1����ͼ�ǵ��ˮʵ��װ�á���ʵ������У��Թ�1������������ ��д��ˮ��ͨ�������·�Ӧ�Ļ�ѧ����ʽ ��
��2����Դˮ����������ˮ�Ĺ������������ʯ�ң���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ__ __��
��3��20��ʱ���Ȼ��Ƶ��ܽ��Ϊ36g����20��ʱ�Ȼ��Ʊ�����Һ�����ʺ��ܼ���������Ϊ ��
��4��Ϊ�˽���ũҵѡ�֣��ֽ�200g30%���Ȼ�����Һϡ��Ϊ10%���Ȼ�����Һ����Ҫ��ˮ������Ϊ ��
��5������ˮ��ͨ��������������ɱ������������������Ũ��ˮ������������豸�����������Ƿ�������й©��A��B��C��D��ʾ4�����ʣ�����ʾ��ͼ���±���A��B��һ�������·�Ӧ����C��D��
| ���� | A | B | C | D |  |
| ��ѧʽ | NH3 | Cl2 | N2 | | |
| ��ʾ��ͼ |  |  |  |  |
��17 g A�μӷ�Ӧ��������C������Ϊ g��
2013 �������������ͻȪ��������������̶�����Ǻӵ�������Դ���齨���µ�һȪ�羰�����������ص���Ȼɽˮ���ۺ�������ʷ�Ļ�������һ�壬Ϊ 5A ��������
��1����ͻȪˮ����__________����������������
��2��ij��ѧС���ͬѧ�Ի��Ǻӵ�ˮ����������ص��о�����Ҫ�� pH ��ֽ���Բⶨ��ˮ�������ǿ�����ⶨ�ľ��巽����
��3��Ϊ�˼��������ˮ����ˮ����Ӳˮ������ˮ���м���___________�����衣
��4��������Ȫˮ֮������Ȫˮ������˵���д������ ������ţ�
| A���峺��Ȫˮ����Һ | B�������Ǿ����̶���ߵľ�ˮ���� |
| C��������еķ�������Ȫˮ��Ӳ�� | D�����˿��Գ�ȥȪˮ�еĿ��������� |
ij��ѧ��ȤС���ͬѧ�������ͼ��ʾ�ļ��ĵ��ˮʵ��װ�ã����м�ͥСʵ�飬������������ݳ�������������±���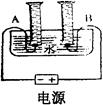
| ʱ�� | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| ���ӵ�Դ��������ͲA������ | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 |
| ���ӵ�Դ��������ͲB������ | 2 | 4 | 6 | 9 | 12 | 15 | 18 | 21 |
��1����ʵ��A��B�����ռ���������ֱ��� ����װ�����������á�������ȷ���������е���������Ȳ�����ȫ�������۱�ֵ�����ܵ�ԭ���� ��
��2�����ʱ�۲쵽A��B���е�Һ����½���ͬʱ���ֹ۲쵽ˮ���е�Һ����������������֪ʶ���ͣ�ˮ����Һ��������ԭ���� ��