��Ŀ����
����Ŀ�����ࡢ��ȡ�Ǩ����ѧϰ��ѧ�ķ�����
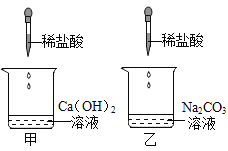
��1����ѧ��չʷ������ʵ��֮�����һ���Ĺ�������Щʵ���ǡ�һ�����ʵ���ʶ���о��������ʵĻ���������Щʵ���ǡ�ҩƷ���о�������ͬ���о�Ŀ�IJ�ͬ�����ݴ˿ɽ�����ʵ���е�_____������ĸ����Ϊһ�࣬������_____��
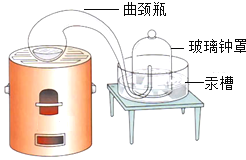
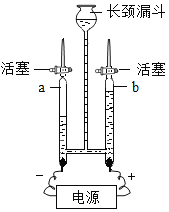
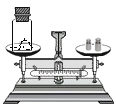
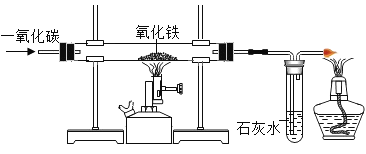
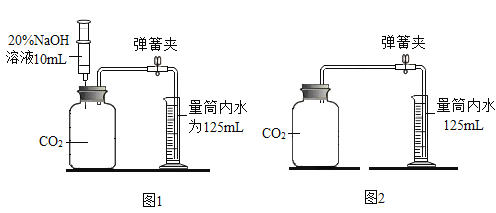
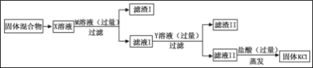
ʵ��Ŀ�� | �о������ɷ� | ̽��ˮ����� | ��ѧ��Ӧǰ�����ʵ�������ϵ | һ����̼��ԭ������ |
ʵ��װ�� |
|
|
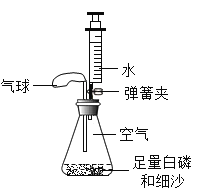
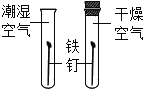
45�����������������ȵõ�41.5�������Ĺ���3.5������������ |
|
A | B | C | D |
��2��ͨ�������Ķ�����Fe3O4�Ļ�ѧʽ�ɿ�����FeOFe2O3��2KAl��SO4��2��ѧʽ�ɿ�����K2SO4Al2��SO4��3ͬ������ȸʯ��Cu2��OH��2CO3���Ļ�ѧʽ�ɿ���_____����д��Fe3O4�����ᷴӦ�Ļ�ѧ����ʽ_____��
���𰸡�AC ҩƷ���о�������ͬ���о�Ŀ�IJ�ͬ ̼��ͭ��CuCO3����������ͭ��Cu(OH)2�� Fe3O4��8HCl=FeCl2��2FeCl3��4H2O
��������
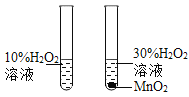
��1���ɽ�ʵ���е�A��C��Ϊһ�࣬�����ǣ�ҩƷ���о�������ͬ���о�Ŀ�IJ�ͬ���������ù���������Ӧ����Aʵ���о������ɷ֣�Cʵ���о������غ㣻
��2����ȸʯ[Cu2��OH��2CO3]�Ļ�ѧʽ�ɿ���̼��ͭ��CuCO3����������ͭ��Cu(OH)2����Fe3O4�����ᷴӦ�����Ȼ��������Ȼ�����ˮ�Ļ�ѧ����ʽ��Fe3O4��8HCl=FeCl2��2FeCl3��4H2O��