��Ŀ����
����Ŀ��ijУ��ѧ��ȤС��ι��Ƽ���������Ϣ���������������о���
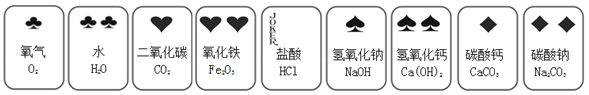
���������ϡ�
����ˮ����ͭ�ǰ�ɫ���壬��ˮ������
������ԭ�ϴ����к�����������������(MgCl2��CaC12)�����������ʡ�
������ԭ��:NaCl+NH3+CO2+H2O=NaHCO3��+NH4C1������þ���A����ʹ�������ȣ��ɷֽ��Ƶô�����ֳ����������
���Ȼ�立ֽ�Ļ�ѧ����ʽ��NH4Cl===NH3��+HCl����
�ݲ���������������ͼ��ʾ:

���������ۡ�
(1)����ҺA�е�������NaCl��__________��______________��
�ڲ����������Ϊ___________��
��д������NaOH��Һ��������Ӧ�Ļ�ѧ����ʽ___________��
��������Na2CO3��Һ�������dz�ȥ�����е�___________��
(2)�������������п�ѭ��ʹ�õ���___________ (�����)��
A.NH3 B. NaOH C. HCl D .CO2
�����̽����
(3)�پ���A���ȷֽ�Ļ�ѧ����ʽΪ ________________________��
�����ʵ����鴿����Ʒ���Ƿ���о���A��������±�(������װ����ѡ��):

ѡ���װ�� | ʵ������ | ʵ����� |
________ | ________ | ��Ʒ��������A |
�����̽������
(4)ȡ������Ʒ��ˮ�ܽ⣬�����Һ�м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ_______________���ɴ�ȷ��������Ʒ��������NaCl��
�����̽������
(5)ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ��:

���Ȼ�����Һ������Ŀ����___________________��
���ж������Ƿ�ϴ�ɾ�����������ϴ��Һ�еμ�________��Ȼ��۲������жϡ�
A.�Ȼ�����Һ B.ϡ���� C.ϡ���� D.̼�����Һ
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ__________��(��ȷ��0. 1 % )
���𰸡� NaOH Na2CO3 ���� MgCl2+2NaOH=Mg��OH��2��+2NaCl ��CaCl2 ACD 2NaHCO3![]() Na2CO3+ H2O+CO2�� ѡ���װ�ã���AB����AC����ACB�� ʵ������B������ǡ���C������ B����� AgNO3+NaCl=AgCl��+NaNO3 ��̼����ȫ����Ӧ BD 88.3%
Na2CO3+ H2O+CO2�� ѡ���װ�ã���AB����AC����ACB�� ʵ������B������ǡ���C������ B����� AgNO3+NaCl=AgCl��+NaNO3 ��̼����ȫ����Ӧ BD 88.3%
��������������ѧ֪ʶ��������Ϣ֪�����������ϡ�����ˮ����ͭ�ǰ�ɫ���壬��ˮ������������ԭ�ϴ����к�����������������(MgCl2��CaC12)�����������ʡ�������ԭ��:NaCl + NH3 + CO2+ H2O = NaHCO3��+ NH4C1������þ���A����ʹ�������ȣ��ɷֽ��Ƶô�����ֳ�������������Ȼ�立ֽ�Ļ�ѧ����ʽ��NH4Cl===NH3��+HCl�����ݲ����������̡á��������ۡ�(1)����ҺA�е�������NaCl��NaOH ��Na2CO3 ���ڲ����������Ϊ���ˡ� �ۼ���NaOH��Һ��������Ӧ�Ļ�ѧ����ʽ�� MgCl2+2NaOH=Mg��OH��2��+2NaCl����������Na2CO3��Һ�������dz�ȥ�����е�CaCl2�� (2)�������������п�ѭ��ʹ�õ��� A.NH3�� C. HCl�� D .CO2�������̽����(3)�پ���A���ȷֽ�Ļ�ѧ����ʽΪ. 2NaHCO3 ![]() Na2CO3+ H2O+CO2���������ʵ����鴿����Ʒ���Ƿ���о���A���v5)ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȡ�
Na2CO3+ H2O+CO2���������ʵ����鴿����Ʒ���Ƿ���о���A���v5)ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȡ�
ѡ���װ�� | ʵ������ | ʵ����� |
��AB����AC����ACB�� | ��B������ǡ���C������ | ��Ʒ��������A |
�����̽������(4)ȡ������Ʒ��ˮ�ܽ⣬�����Һ�м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪAgNO3+NaCl=AgCl��+NaNO3 ���ɴ�ȷ��������Ʒ��������NaCl�������̽������(5)�ⶨ�ô�����Ʒ�Ĵ��ȡâ��Ȼ�����Һ������Ŀ����̼����ȫ����Ӧ�����ж������Ƿ�ϴ�ɾ�����������ϴ��Һ�еμ�B.ϡ����D.̼�����Һ��Ȼ��۲������жϡ��۽������Ʒ��̼���Ƶ���Ϊxg��
BaCl2��Na2CO3��BaCO3����2NaCl
106 197
X 19.7g
![]() ��
��![]() , x��10.6g
, x��10.6g
Ʒ��̼���Ƶ���������Ϊ��![]() ��100����88.3��.
��100����88.3��.
�㾦�ñ�����Ҫ��������ᴿ�����鴿����Ʒ�ɷ֣��ⶨ�ô�����Ʒ�Ĵ��ȵ�֪ʶ��