��Ŀ����
����Ŀ����������һƿ�ܵ�ͨ����Ʒ��ʶ���±���
| ��Ʒ�� | ����ܵ���ͨ�� |
��Ҫ�ɷ� | ��������60% ̼����15% | |
ע������ | ��Ҫ��Ƥ���Ӵ� | |
���� | 600g |
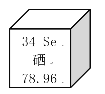
С�����ⶨ��ʶ��̼���Ƶĺ����Ƿ���ʵ����������ʵ�飺ȡ10g�ܵ�ͨ��Ʒ����������ˮ������ȫ��ȴ����ȥ���������Һ�м����������Ȼ�����Һ������ȫ��Ӧ���ˣ�ϴ�ӡ���ɣ�������������Ϊ2g��(����ܵ�ͨ�����ɷֲ��μӻ�ѧ��Ӧ)����ͨ������˵��̼���Ƶı�ʶ�Ƿ���ʵ___(��д���������)
���𰸡���
�⣺��10g�ܵ�ͨ��Ʒ�к�̼���Ƶ�����Ϊx��

![]()
x=2.12g��
Na2CO3%=![]() ��100%=21.2%
��100%=21.2%
��21.2%��15%������̼���Ƶĺ�����ʶ����ʵ��
��������
�⣺��10g�ܵ�ͨ��Ʒ�к�̼���Ƶ�����Ϊx��

![]()
x=2.12g��
Na2CO3%=![]() ��100%=21.2%
��100%=21.2%
��21.2%��15%������̼���Ƶĺ�����ʶ����ʵ��

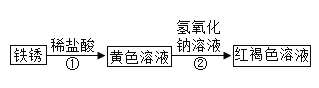
����Ŀ��һ��,ʵ������С���߽�ʵ���Һ���ʦһ����ÿ��ʵ�����ϵ�ҩƷ�������Ƿ��뱸,�ߵ�ij���ʱ��,������һ������г����������(��ͼ).
��1������˾��������뵽���������ܱ����ˣ�д���ñ��ʷ�Ӧ�Ļ�ѧ����ʽ��__________________
��2��Χ�ƴ�ƿNaOH��Һ�Ƿ���ʵ�����,С������ʵ���ҵ������Լ�(�Ȼ�����Һ��ϡ���ᡢ��̪��Һ)չ����̽�����
��ȡ������Һ���Թ��У��μ�ij���Լ��������ݲ������ɴ�֤��NaOH��Һ�Ѿ����ʡ�����ΪС�����ӵ��Լ���_____________��
����֤�����ʵ���Һ���д�NaOH���������С���������̽��������
̽��Ŀ�� | ̽������ | Ԥ������ |
������Һ�е�CO32- | aȡ������Һ���Թ���,�μ�������_______�Լ� | �а�ɫ�������� |
֤����Һ���д�NaOH | b��ʵ���������Һ�еμӷ�̪��Һ | ___________ |
��3��ͨ������̽����˵������������Һ��¶�ڿ�������________��Ӧ���ʣ���Ӧ��________���档
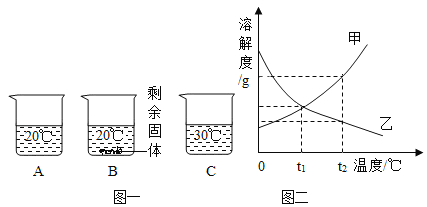
����Ŀ��ij��ѧ��ȤС����̽��ϡ����Ļ�ѧ����ʱ��������ͼһ��ʾ������ʵ�飮
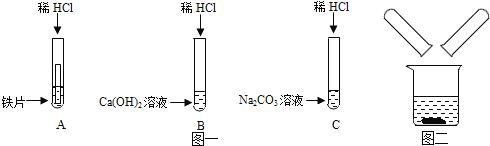
��1��д��A�Թ��еķ�Ӧ����_____��
��2��д��B�Թ��з�Ӧ�Ļ�ѧ����ʽ_____��
��3��ʵ�������С��ͬѧ��_____��֧�Թ��еķ�Һͬʱ����һ���ྻ���ձ��У���ͼ����ʾ�����۲쵽�������ݲ��������а�ɫ�������ɣ�ͬѧ�ǽ��ձ��ڵ����ʽ��й��ˣ���������Һ�����ʳɷֽ���̽����
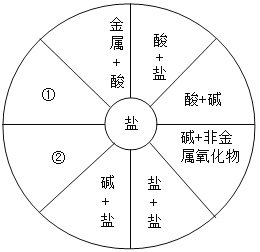
��������룩
��ͬѧ���룺NaCl�� ��ͬѧ���룺NaCl��CaCl2��
��ͬѧ���룺NaCl��CaCl2��HCl�� ��IJ��룺_____��
���������ۣ�
����Ϊ_____ͬѧ�IJ���һ������ȷ��������_____��
�����ʵ�飩�����ʵ��֤����IJ�����ȷ��
ʵ�鲽�� | ʵ������ | ʵ����� |
ȡ������Һ���Թ��У������еμ�_____ | _____ | ������� |