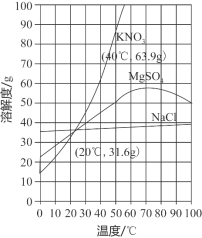
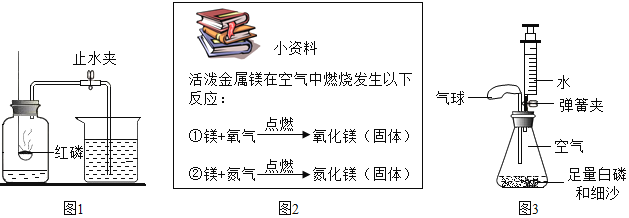
��Ŀ����
����Ŀ��С��ͬѧ������ʵ������˸Ľ����������£�
�������İ������ݻ�Ϊ40mL���Թ��У������������Թܣ�ͨ��������ʵ���ݻ�Ϊ60mL�����Ժܺõ�ע�������ӣ���ͼ��ʾ��������ռ������Բ��ƣ���
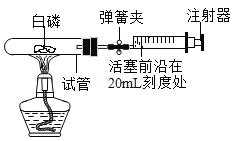
��1��ʵ��ǰ�����ɼУ���ע����������ǰ�ش�30mL�̶ȴ�����20mL�̶ȴ������ֺ����������ܷ�����______mL�̶ȴ�����˵��_________��
��2���н����ɼУ����Ȱ���ʹ���ַ�Ӧ���Թ���ȴ�����ɿ����ɼУ����������ƶ�������ͣ����________mL����
��3������ʹ�õ��ɼУ����Ȱ��׳�ַ�Ӧ����ȴ���ɹ۲쵽��������_____��ѡ�������������������ƶ���ԭ����______�����ջ����ȶ���________mL����
��4������С����������ʵ�飬ʵ�ʿ�������������������Σ�գ����ʵ��ʧ�ܡ����磺______��
��5��С��ͬѧҲ������ʵ������˸Ľ����������滻����������вⶨ�������������������ʵ�顣����Ϊ���������������ȡ�÷��㡣������С��Ľ�ʵ��Ŀ����Ժ�ȷ�ԡ�
������������____________��ȷ��������____________��
���𰸡�30mL װ�������������� 22mL �� ����ȼ�ղ�������ʹ�Թ��ڿ������� 16mL ����ȼ�գ������Ѹ�����ͣ���ѹ������ʹ�Թܵ���Ƥ���������ע���������������Ҳ���ܵ����Թ����ѡ� ����ȼ����Ȼ����������Ӧ��������������ͬʱ�ֲ���������̼���壬ʹƿ����ѹ�仯���������������滻������ʵ�鲻���С� ��Ϊ������̼������ˮ�����ܽ�����Ƚ��٣����ɿ����ɼ�ʱ������������ˮ����������ƿ�У�ˮ������������������������Ƚϴ����������ַ������������ȷ��
��������
�����Ƕ���һ�����ʵ��Ľ�������ע�����Ķ������жϿ����������ĺ�����
��1��ʵ��ǰ�����ɼУ���ע����������ǰ�ش�30mL�̶ȴ�����20mL�̶ȴ������ֺ����������ܷ�����30mL�̶ȴ�����˵��װ�������������á�
��2������ȼ�������������������������������Լռ���������1/5���м��㣺![]() �����������ƶ�8mL������ͣ����20mL -8mL=12 mL����
�����������ƶ�8mL������ͣ����20mL -8mL=12 mL����
��3������ʹ�õ��ɼУ����Ȱ���ȼ�գ���������ʹ�Թ��ڿ������ͣ�ѹǿ���ɹ۲쵽�����������ƶ������Թ���ȴ�����������ģ�ѹǿ��С�������������ƶ�����ʱ�������������Թ��ڵ�������ע�����ڵ����������Ϊ��![]() �����ջ����ȶ���30mL-14 mL=16 mL����
�����ջ����ȶ���30mL-14 mL=16 mL����
��4������ȼ�գ������Ѹ�����ͣ���ѹ������ʹ�Թܵ���Ƥ���������ע���������������Ҳ���ܵ����Թ����ѡ���5������ȼ����Ȼ����������Ӧ��������������ͬʱ�ֲ���������̼���壬ʹƿ����ѹ�仯���������������滻������ʵ�鲻���У���Ϊ������̼������ˮ�����ܽ�����Ƚ��٣����ɿ����ɼ�ʱ������������ˮ����������ƿ�У�ˮ������������������������Ƚϴ����������ַ������������ȷ��

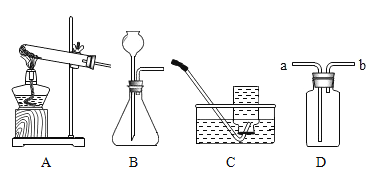
 ��У����ϵ�д�
��У����ϵ�д�����Ŀ������ʵ������ܴﵽĿ�ĵ��ǣ�������
A | B | C | D |
|
|
|
|
֤��������̼����ȼ������ȼ���ܶȴ��ڿ��� | �ⶨ�����������ĺ��� | ֤����ȼ��ȼ����Ҫ�������¶ȴﵽ�Ż�� | ֤����������ˮ��������ͬ���õĽ�� |
A.AB.BC.CD.D
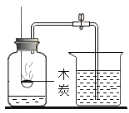
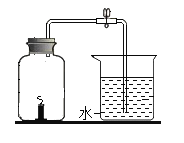
����Ŀ��Ϊ̽��̼��ԭ����ͭ�����ʵ��������ȡľ̿�ۺ�����ͭ�ĸ�������1-2.5g����ͼ��ʾװ�ý���ʵ�顣
(1)ľ̿��ԭ����ͭ�Ļ�ѧ����ʽΪ__��ʵ�������Aװ�����Թ��ڲ�����������__��
(2)�ƾ��ƻ���ӽ������ֵ�Ŀ����____��
���������ϣ�������ͭ(CuO)Ϊ��ɫ���塣��̼��ԭ����ͭ�õ���ͭ�п��ܺ���������������ͭ��������ͭΪ��ɫ���壬����ϡ���ᷴӦ��Cu2O+H2SO4=CuSO4+H2O+Cu
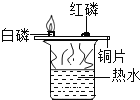
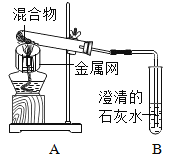
���������飩ȡһ�����Ļ�������ͼ��ʾװ�ý���ʵ�顣
��� | ľ̿��������ͭ�������� | ��Ӧ�����ʵ���ɫ��״̬ | |
1 | 1��9 | ��ɫ�����н������� | ����������ɫ���� |
2 | 1��10 | ���к�������ɫ���� | |
3 | 1��11 | ���м�������ɫ���� | |
4 | 1��12 | ��ɫ���� | |
5 | 1��13 | ���н϶��ɫ���� | |
����������ͣ�(3)������ʵ����Եõ��Ľ�����___��������ѡ�
����˼�����ۣ�
(4)Ϊ�˼������ɵĺ�ɫ�������Ƿ���Cu2O�������Լ���____��
(5)��װ����̲��е�װ����������Ľ���ʵ�����ʱ�������õ��ɼмн���Ƥ�ܣ���Ϩ��ƾ��ƣ���������Ŀ�ij��˷�ֹʯ��ˮ�������ȵ��Թܣ�ʹ�Թ�ը���⣬�����Է�ֹ____��