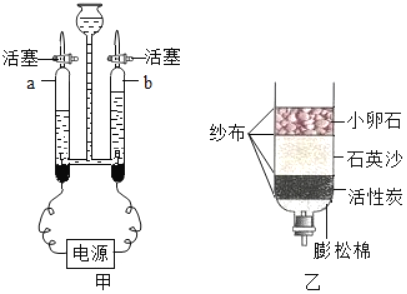
��Ŀ����
����Ŀ����ͼ��ʾ���dz��õ�ʵ��װ�ã���ش��������⣺
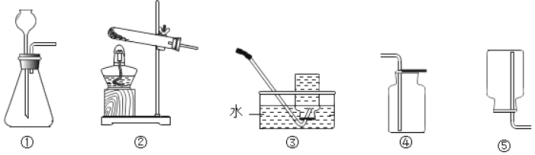
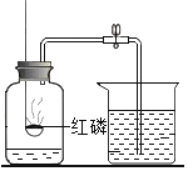
��1���ֽ���������ȡ������Ӧѡ�õķ���װ����_____������ţ�������Ҫ_____������ʱӦע��_____��
��2������װ�â���ȡ����������©���м����Լ��Ļ�ѧʽ��_____����ƿ�м����Լ��Ļ�ѧʽ��_____��
��3������װ�â��ռ����������ַ�������_____������������_____��������_____����ʱ�����ռ�������
��4������װ�â��ռ������������������ռ����ķ�����_____��
���𰸡�B�� ���Թܿڷ�һ������ �Թܿ�Ӧ��������б�� H2O2�� MnO2�� ��ˮ���� ������������ˮ�� ���ݴ�ƿ���ݳ�ʱ�� �Ѵ����ǵ�ľ�����ڼ���ƿ�ڣ��������ǵ�ľ����ȼ��˵���Ѿ��ռ�
��������
��1���ֽ���������ȡ���������ڹ�������ͣ�Ӧ��ѡ�õķ���װ����B������Ҫ���Թܿڷ�һ��������ֹ����ʱ������ط�ĩ���뵼�ܣ�����ʱӦע���Թܿ�Ӧ��������б����ֹ����ˮ�������ȵ��Թܵײ���ը���Թܣ�
��2����װ�â���ȡ���������ڹ�Һ�����ͣ����õķֽ����������Һ�ķ���������©���м�����Լ��ǹ������⣬�仯ѧʽΪH2O2����ƿ�м����Լ��Ƕ������̣��仯ѧʽΪ��MnO2��
��3������װ�â��ռ����������ַ���������ˮ������Ϊ������������ˮ�����������ݴ�ƿ���ݳ�ʱ�������ռ�������
��4��������֧��ȼ�գ���װ�â��ռ������������������ռ����ķ����ǰѴ����ǵ�ľ�����ڼ���ƿ�ڣ��������ǵ�ľ����ȼ��˵���Ѿ��ռ�����

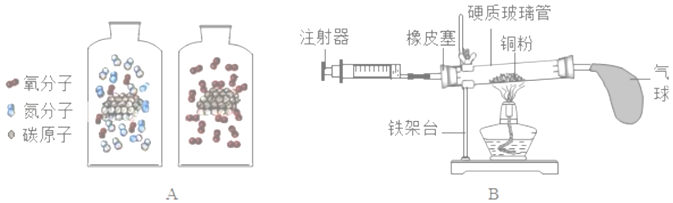
����Ŀ����ѧ���о���ѧϰ����Ҫ������������ģ�ͣ������ú��ʵĻ�ѧ����Ϊ���ߣ������о��ͱ�����ۻ�ѧ���ʵ����ʺͱ仯��
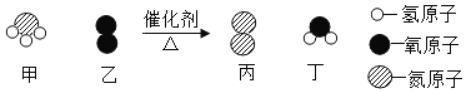
��һ����ѧ����ʽ����д��ѧ�û�ѧ�Ĺؼ������Ǻܶ�ͬѧ�ڸո�ѧϰ��ѧ����ʽ��дʱ�������᷸һЩ������������£�
�������ﻯѧʽ��д�����δ��ƽ����©��Ӧ�����ܡ���������ʹ�ò��������ж����л�ѧ����ʽ�Ĵ������ͣ�����ţ���
��� | ��ѧ����ʽ | �������� |
��1�� | þ��ȼ�գ� | ______ |
��2�� | ������ͭ��Һ������������Һ��ϣ�CuSO4+NaOH==Na2SO4+Cu(OH)2�� | ______ |
��3�� | ��¯ұ�������� | ______ |
��������ѡ�á�>����<����=����ա�
��4���Ͻ��Ӳ�ȣ�һ������£��Ͻ�______��ɷֽ�����
��5��ͨ��״���µ�Һ�������100mL�ƾ���100mLˮ��Ϻ�����_____200mL��
��6��1g�����1g��������ȫȼ�պ����ɶ������������______2g��
��������7��������ȼ���йص�ʵ���У�С��ͬѧ���ֺ����Ż����240�棬�������Ż����40�档�ɴ����ǿ����жϣ�����һ��Ԫ����ɵ����ʽ������ʡ����˵����©������˵�����ɣ�____________��