��Ŀ����
����Ŀ����ѧ�о���ѧϰС���ⶨ����������������ijɷ���Ϊ���⣬���ǽ��ռ��������̶���ˮ�۵ײ�������ƺ���___________���ռ�һƿ�������塣
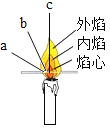
����ͼ��ʾ��װ�ý���ʵ�飺��ȼȼ�ճ��ڵĺ�����������ƿ�в�������������������Ϩ����ȴ���ɼУ�����ƿ�е�ˮ�����Ϊ����ƿ�ݻ���1/4��˵������������һ������___________����������ʵ�飬���ԱȽ�����������ɷֵĺ��������������ɷֺ������������_________��
���𰸡���ˮ�� ���� ���������������ȿ���������������
��������
�ռ������е���������ˮ�ۣ����Ʋ��ռ���������ˮ����
����������������ȼ�գ����Ʋ������к���������
���ݼ���ƿ�е�ˮ�����Ϊ����ƿ�ݻ���1/4�������ˮ��1/5�֤࣬�����������������ȿ����ж���

����Ŀ�������±��ش����⡣
�¶ȣ����� | 20 | 40 | 50 | 60 | 80 | |
�ܽ�ȣ�g/100g ˮ�� | NaCl | 36.0 | 36.6 | 37.0 | 37.3 | 38.4 |
NH4Cl | 37.2 | 45.8 | 50.4 | 55.2 | 65.6 | |
KNO3 | 31.6 | 63.9 | 85.5 | 110 | 169 | |
��1��20��ʱ�����������ܽ�ȴӴ�С��˳����__________��
��2��������к����������Ȼ������ʣ��ɲ���________���ᴿ����ء�
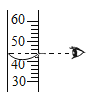
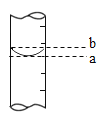
��3����Ͳ�ľֲ�ʾ��ͼ����ͼ������ȡˮʱӦ����____���ߣ�ѡ����a������b�������ж�����____���ߣ�ѡ����a������b������Ӧ�Ķ����ϴ�
��4��A��80������120 gˮ��KNO3��Һ���������²������õ�102 gKNO3���塣
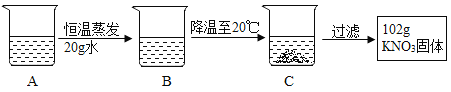
��.A��ҺΪ___________��ѡ��������������������������Һ��
��.�����Ϲ��̵ķ���������ȷ����__________��ѡ���ţ���
a.A��B�Ĺ����У���������û�иı�
b.B���������ܼ���������Ϊ169�U100
c.��ʼ����KNO3������¶���60����80��֮��
d.��Һ����������222 g
����Ŀ����ѧ��һ����ʵ��Ϊ��������Ȼѧ�ƣ�ʵ�����Ҫ�淶��ȷ������ʵ�������������ȷ����
ʵ �� �� �� ͼ ʾ |
|
|
|
|
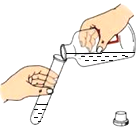
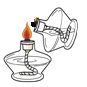
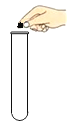
ѡ �� | A. ȡ��Һ��ҩƷ | B. ��Ͳ������� | C. ���ƾ��Ƶ�ȼ | D. �����״���� |
A.AB.BC.CD.D