题目内容
某校化学兴趣小组同学发现,长期使用的热水壶底部有一层水垢,水垢的主要成分是碳酸钙和氢氧化镁.他们为了测定水垢中碳酸钙的含量,将足量质量分数为10%的盐酸加入到10g水垢中,产生CO2气体的情况如图所示.
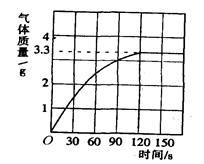
(1) 从图中可以看出,10g水垢与盐酸反应生成的二氧化碳最多是________ g。
(2) 水垢中碳酸钙的质量是________ g。
(3) 除去水垢中碳酸钙至少需要质量分数为10%的盐酸的质量是________ g (最后结果保留一位小数).
(4)写出与本实验有关的化学方程式
1、_______________________________________________________________
2、_______________________________________________________________

(1) 从图中可以看出,10g水垢与盐酸反应生成的二氧化碳最多是________ g。
(2) 水垢中碳酸钙的质量是________ g。
(3) 除去水垢中碳酸钙至少需要质量分数为10%的盐酸的质量是________ g (最后结果保留一位小数).
(4)写出与本实验有关的化学方程式
1、_______________________________________________________________
2、_______________________________________________________________
(1)3.3 (1分) (2)7.5 (2分) (3)86.2g(2分)
1、CaCO3 + 2HCl ="=" CaCl2 + H2O + CO2↑ (2分)
2、Mg(OH)2 + 2HCl ="=" MgCl2 + 2H2O
1、CaCO3 + 2HCl ="=" CaCl2 + H2O + CO2↑ (2分)
2、Mg(OH)2 + 2HCl ="=" MgCl2 + 2H2O
:(1)由图可知,120s后恰好完全反应,放出二氧化碳3.3g;
(2)设放出3.3g二氧化碳需要碳酸钙的质量为x,消耗HCl的质量为y
CaCO3+2HCl═CaCl2+H2O+CO2↑
100 73 44
x y 3.3g
100÷44= x÷ 3.3 解得:x=7.5g
73÷44= y ÷ 3.3 解得:y=5.475g
(3)氢氧化镁完全反应消耗HCl质量为z
Mg(OH)2+2HCl═MgCl2+2H2O
58 73
10g-7.5g z
58÷73=(10g-7.5g )÷z 解得:z≈3.147g
至少需要质量分数为10%的盐酸的质量=(5.475g+3.147g)÷10%≈86.2g
(4)盐酸即能与碳酸钙反应也能与氢氧化镁反应,其反应的方程式分别为:
CaCO3+2HCl═CaCl2+H2O+CO2↑;Mg(OH)2+2HCl═MgCl2 +2H2O;
(2)设放出3.3g二氧化碳需要碳酸钙的质量为x,消耗HCl的质量为y
CaCO3+2HCl═CaCl2+H2O+CO2↑
100 73 44
x y 3.3g
100÷44= x÷ 3.3 解得:x=7.5g
73÷44= y ÷ 3.3 解得:y=5.475g
(3)氢氧化镁完全反应消耗HCl质量为z
Mg(OH)2+2HCl═MgCl2+2H2O
58 73
10g-7.5g z
58÷73=(10g-7.5g )÷z 解得:z≈3.147g
至少需要质量分数为10%的盐酸的质量=(5.475g+3.147g)÷10%≈86.2g
(4)盐酸即能与碳酸钙反应也能与氢氧化镁反应,其反应的方程式分别为:
CaCO3+2HCl═CaCl2+H2O+CO2↑;Mg(OH)2+2HCl═MgCl2 +2H2O;

练习册系列答案
 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案
相关题目
 2CuO + H2O +
2CuO + H2O + ↑,“
↑,“  CaO + CO2 ↑ ,测得反应后固体的质量(m)与反应时间(t)的关系如下表:
CaO + CO2 ↑ ,测得反应后固体的质量(m)与反应时间(t)的关系如下表: