��Ŀ����
��8�֣������������н϶�ơ�þ���ӵ�ˮ���������ʯ��֢�����ȿ�ʹˮ�иơ�þ����ת��Ϊ������ˮ����������Ҫ�ɷ���CaCO3��Mg(OH)2��Ϊȷ��ˮ���ijɷ֣����ʵ��С�����������ʵ�飺
�ٳ�ȡ5gˮ����Ʒ��������100mL�ձ��У�Ȼ��ע��50 g 10%��ϡ���
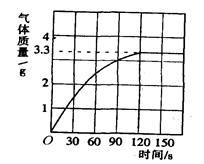
�ڷ�Ӧֹͣ��Ӧ����������������1.54g��
����ȡ����ˮ������ʵ��ڵĻ��Һ�У������ݲ�����
�����������Ϣ�ش�
��1��ʵ��۵�Ŀ���� ��
��2������ˮ����Ʒ��̼��Ƶ�����������Ҫ����ݻ�ѧ����ʽ���㣬��������ȷ��0.01����
�ٳ�ȡ5gˮ����Ʒ��������100mL�ձ��У�Ȼ��ע��50 g 10%��ϡ���
�ڷ�Ӧֹͣ��Ӧ����������������1.54g��
����ȡ����ˮ������ʵ��ڵĻ��Һ�У������ݲ�����
�����������Ϣ�ش�
��1��ʵ��۵�Ŀ���� ��
��2������ˮ����Ʒ��̼��Ƶ�����������Ҫ����ݻ�ѧ����ʽ���㣬��������ȷ��0.01����
��1��֤��ˮ����ȫ��Ӧ������ʣ�ࡣ ��2��70%
CaCO3��ϡ���ᷴӦ�ų�CO2�����������غ㶨�ɷ�Ӧ�������������ٵ�1.54gΪ������̼����ȡ����ˮ������ʵ��ڵĻ��Һ�У������ݲ���˵�����������ˮ���е�̼�����ȫ��Ӧ��
(2) ���������غ㶨��֪���ɶ�����̼������Ϊ1.54g
�⣺��ˮ����̼��Ƶ�����Ϊx
CaCO3 + 2HCl === CaCl2 + H2O + CO2��
100 44
X 1.54g
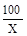 =
=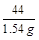
X=3.5g
ˮ����Ʒ��̼��Ƶ���������Ϊ��
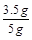 ��100%=70%
��100%=70%
��ˮ����Ʒ��̼��Ƶ���������70%
(2) ���������غ㶨��֪���ɶ�����̼������Ϊ1.54g
�⣺��ˮ����̼��Ƶ�����Ϊx
CaCO3 + 2HCl === CaCl2 + H2O + CO2��
100 44
X 1.54g
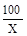 =
=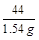
X=3.5g
ˮ����Ʒ��̼��Ƶ���������Ϊ��
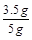 ��100%=70%
��100%=70%��ˮ����Ʒ��̼��Ƶ���������70%

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ