��Ŀ����
����Ŀ�����⻯ѧ��ȤС���ͬѧ����ij�������ķϼ�Һ����Ҫ�ɷ�ΪNa2CO3������������NaCl����ʯ�������������Ƶ�����Һ��Ϊԭ���Ʊ��ռ�������õ��ռ�ֲ�Ʒ�ijɷֽ��з����Ͳⶨ��
���������ϡ��ټ�ʯ����CaO��NaOH�Ĺ�����������ն�����̼��ˮ��
��CO2+Ba��OH��2 �T BaCO3��+H2O��
���ֲ�Ʒ�Ʊ���
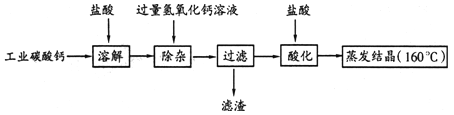
��1�����ϼ�Һ��������Ũ�����γɽ�Ũ����Һ����ȴ����ʯ�����ϣ�������Ӧ�Ļ�ѧ����ʽΪ ��
��2������Ӧ��Ļ������ˣ��õ�����Һ���������ᾧ���Ƶ�NaOH�ֲ�Ʒ��
���ֲ�Ʒ�ɷַ�����
��1��ȡ�����ֲ�Ʒ����ˮ�ó�����Һ������Ba��NO3��2��Һ���ְ�ɫ�������ɴ˸ôֲ�Ʒ��һ�������� ��������
��2����С��ͬѧͨ���Դֲ�Ʒ�ɷֵ�ʵ�������ȷ���ôֲ�Ʒ�к����������ʡ�
�������ⶨ��Na2CO3�����IJⶨ��
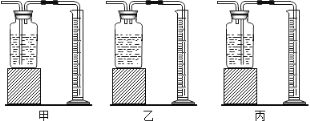
����ȤС���ͬѧ�������ͼ��ʾ��ʵ��װ�á�ȡ20.0g�ֲ�Ʒ������ʵ�顣
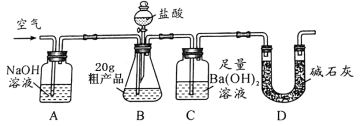
��1��ʵ����������������ͨ���������Ŀ���� ��
��2�����и����ʩ�У���Ӱ���ⶨȷ�ȵ���______����������
a���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
b���μ�����˹���
c����A-B֮������ʢ��Ũ�����ϴ��װ��
d��ȡ����ͼ�У�װ��
��3��ʵ����ȷ��ȡ20.0g�ֲ�Ʒ�����вⶨ�����BaCO3����Ϊ1.97g����ֲ�Ʒ��̼���Ƶ���������Ϊ ��
������������Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ���� ��
���𰸡����ֲ�Ʒ�Ʊ�����1��Na2CO3 +Ca��OH��2 = CaCO3�� + 2NaOH
���ֲ�Ʒ�ɷַ�������1��Ca��OH��2
���Ͽ�֪�ֲ�Ʒ��һ����̼���ƣ���̼������������������Һ�в��ܹ���
�������ⶨ��Na2CO3�����ⶨ�������ɵ�CO2����ȫ������C�У�ʹ֮��ȫ��Ba��OH��2��Һ����
��2��c ��3��5.3% ��4��B�е�ˮ�������Ȼ�������Ƚ���װ��C��
��������
������������ֲ�Ʒ�Ʊ�����1�����ϼ�Һ��������Ũ�����γɽ�Ũ����Һ���������溬��̼������Һ������ȴ����ʯ�����ϣ�������Ӧ�Ļ�ѧ����ʽΪ��Na2CO3 +Ca��OH��2 = CaCO3�� + 2NaOH
���ֲ�Ʒ�ɷַ�������1��ȡ�����ֲ�Ʒ����ˮ�ó�����Һ������Ba��NO3��2��Һ���ְ�ɫ������˵����Һ��һ������̼���ƣ��ɴ˸ôֲ�Ʒ��һ�������У�Ca��OH��2 �������ǣ����Ͽ�֪�ֲ�Ʒ��һ����̼���ƣ���̼������������������Һ�в��ܹ���
�������ⶨ��Na2CO3�����IJⶨ����1��ʵ����������������ͨ���������Ŀ���������ɵ�CO2����ȫ������C�У�ʹ֮��ȫ��Ba��OH��2��Һ���գ��Է�������̼���������ƫС
��2��Ũ����ֻ�dz�ȥ�����е�ˮ�֣��Ա�ʵ��û��Ӱ�죬����A-B֮������ʢ��Ũ�����ϴ��װ����Ӱ���ⶨȷ������ѡc
��3�����ݷ�����Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ��Na2CO3 + 2HCl �T 2NaCl + CO2�� + H2O ��CO2+Ba��OH��2 �T BaCO3��+H2O������ֱ���ҳ�Na2CO3��BaCO3��������ϵ�������Na2CO3����������һ��������ֲ�Ʒ��̼���Ƶ���������
�⣺���ֲ�Ʒ��Na2CO3����������Ϊx
����Na2CO3 + 2HCl �T 2NaCl + CO2�� + H2O ��CO2+Ba��OH��2 �T BaCO3��+H2O
�ɵ�: Na2CO3 ~ BaCO3
106 197
x 1.97g
106��197=x:1.97
x = 1.06g
̼���Ƶ���������=1.06g/20g��100%=5.3%
��̼���Ƶ�����������5.3%
��4��������Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����B�е�ˮ�������Ȼ�������Ƚ���װ��C��

����Ŀ���������ʵ���;�����ʵĶ�Ӧ��ϵ��ȷ����
���� | ��; | ���� |
A ������ | ����� | ����ˮ��Ӧ |
B ϡ���� | ������ | ���ӷ� |
C ��� | ������ | ͨ����ܷ�����ɫ�� |
D ���ʯ | �и�� | �ȶ��� |
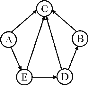
����Ŀ����7�֣�ijͬѧ��ʵ���ҷ�����һƿ��ǩ��ȱ����ɫ��Һ�v��ͼ����ʾ�w��Ϊȷ�����е����ʣ�����Ʋ�����������̽�������ش��������⡣
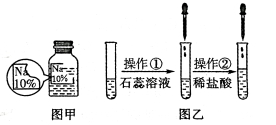
����������衿�����ʿ���ΪNaCl��NaOH��Na2CO3��NaHCO3�е�һ�֡�
�����ϲ��ġ������������ʵ������Ϣ���£�
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�����µ��ܽ�ȣ�g | 36 | 109 | 21.5 | 9.6 |
������ijϡ��Һ��pH | 7 | 13 | 11 | 9 |
��̽�����̡�
��ͼ����ʾ���ڲ����ٺ��ȷ�����ʲ���NaCl������ʵ������Ӧ�� ��
�ڽ��в�����ʱ����ɫ��ζ�õ�����������ɴ��ֿ��ų����������е� ��
��̽�����ۡ�
����Ϊ����Һ�е����ʿ������������������е� ������ж������� ��
��̽����˼��
��1��������̽����������ȷ�ģ������ڲ���������Ӧ�� ��д��ѧʽ����ʵ���Ҽ���������ʵ������������� ��
��2������ͬѧ�������е����ʻ�������Na2SO4������û��Na2SO4������������Ϣ��
�����������ʵ���������������жϸ���Һ�е������Ƿ���Na2SO4�����������ɣ� ��