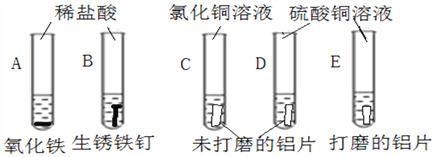
��Ŀ����
����Ŀ�����˽����ȼ����������⣬�����������Ԫ���Ի�����(����������)����ʽ��������Ȼ�硣�����������еĽ�����ԭ�����Ĺ����ڹ�ҵ�ϳ�Ϊ������ұ�������ý�����ԵIJ�ͬ�����Բ��ò�ͬ��ұ��������
һ���ȷֽⷨ����һЩ�dz������ý���������ֱ���ü��ȷֽ�ķ������仯�����л�ԭ���������磺2HgO![]() 2Hg+O2����2Ag2O
2Hg+O2����2Ag2O![]() 4Ag+O2����
4Ag+O2����
������ⷨ����һЩ�dz����õĽ���������һ��Ļ�ԭ�����ѽ����ǻ�ԭ��������ҵ�ϳ��õ�ⷨұ�������磺MgCl2(����)![]() Mg+Cl2���� 2NaCl(����)
Mg+Cl2���� 2NaCl(����)![]() 2Na+Cl2����
2Na+Cl2����
�����Ȼ�ԭ�����ֽ�����ұ������ͨ���ڸ����·�����������ԭ��Ӧ����ɵģ����õĻ�ԭ���н�̿��һ����̼�������ȡ�һЩ���ý���Ҳ������ԭ��������Բ����õĽ������仯�������û�������
����������Ϣ�ش��������⣺
��1���������ȷֽⷨ�ƵõĽ�����______________��
A Cu B Fe C Ag D Hg
��2�����з�Ӧ�����Ϲ�ҵұ������ʵ���������_____________��
A2MgO![]() 2Mg+O2�� B 4CO+Fe3O4
2Mg+O2�� B 4CO+Fe3O4![]() 3Fe+4CO2
3Fe+4CO2
C 2Al+Fe2O3![]() Al2O3+2Fe D 2KCl(����)
Al2O3+2Fe D 2KCl(����)![]() 2K+Cl2��
2K+Cl2��
��3������Ǧ�Ŀ�����ұ��Ǧ����ʹ�����������е�__________��������__________________��
��4����������Ȼ��ƿ��Եõ������ƣ����ǵ��ʳ��ˮ���ܵõ������ƣ���õ�ⱥ��ʳ��ˮ��������Һ��pH����7�����֪����һ����__________����������������嵥�����ɡ�д����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ__________________��
���𰸡�AB A �Ȼ�ԭ�� Ǧ��һ�ֽϻ��õĽ�������Խ�������֮ͭ�� �������� 
��������
��1���������Ϣ��֪����һЩ�dz������ý�������������������ֱ���ü��ȷֽ�ķ������仯�����л�ԭ������ͭ��������Ի��ý���������ͭ�����������ȷֽⷨ�Ƶã����AB��
��2��A���Ʊ�����þ�����õ���Ȼ�þ�ķ������ʴ���
B���������Բ���һ����̼�ڸ��µ������»�ԭ����������ķ���������ȷ��
C��һЩ���ý���Ҳ������ԭ��������Բ����õĽ������仯�������û�����������ȷ��
D���������Ϣ��֪��������ڵ��Ȼ��ؿ��Եõ������غ�����������ȷ��
��ѡ��A��
��3��Ǧ��һ�ֽϻ��õĽ�������Խ�������֮ͭ�䣬�������Ȼ�ԭ����Ǧ�Ŀ�����ұ��Ǧ��
��4����ⱥ��ʳ��ˮ��������Һ��pH����7�����֪����һ���������������ɣ�������������嵥�����ɣ����������غ㶨�ɣ���Ӧ����Ԫ�ػ��ϼ������н�ԭ��֪�������嵥�������������������ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ��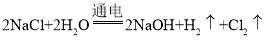 ��
��

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д�����Ŀ����Һ�����ǵ�����ϢϢ��ء�
��1�������������ʷֱ����ˮ�У���ֽ��裬���Եõ���Һ����_____������ĸ����
A ������� B ���� C ��� D �ⵥ��
��2���������ơ�̼���Ʒֱ���ˮ���ƾ��е��ܽ�������ʾ
�������� | ̼���� | ||||
20�� | 40�� | 20�� | 40�� | ||
ˮ | 109g | 129g | 21.8g | 49g | |
�ƾ� | 17.3g | 40g | ���� | ���� | |

������ͼ���ʾ_____��������������������̼�����������ܽ�����ߡ�
��20��ʱ���������Ƶ��ܽ��_____����������������С������̼���Ƶ��ܽ�ȡ�
��40��ʱ���ֱ�50gNaOH�ֱ�Ͷ�뵽100gˮ��100g�ƾ��У����γɱ�����Һ����_____��ѡ����ˮ�������ƾ��������ٽ�����CO2ͨ������NaOH�ľƾ���Һ�У��۲쵽������Ϊ_____����Ӧ�Ļ�ѧ����ʽΪ��_____��
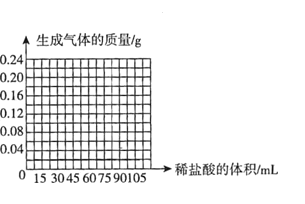
����Ŀ����̺�ϲ���������Ա�������������������һö���ָ�����г��ļ۸�Ҫ���˺ܶ࣬���ȸ����ֵ��Ľ�ָ�Ǽٵġ����������������ڵĻ�ѧ��ȤС���ͬѧ�����ⶨ��ָ����١� �������Ͽ�֪:�ٽ��׳ƻ�ͭ����������ɫ�������Ͽ�����ƽ�Ϊ���ƣ����Ժ������֣���Zn��Cu�Ͻ������Au�������ǻ�ͭ�е��������ʣ���������ƽ�ϳ�����ö���ָ����15g,���ý��ָ�����ձ��У�����Ͳ��ȡ75 mLϡ�������μӵ����У�ÿ�γ�ַ�Ӧ�ⶨ����������������ʵ�����������
��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� | |
����ϡ��������/mL | 15 | 15 | 15 | 15 | 15 |
��������������/g | 0. 04 | 0. 04 | 0. 04 | N | 0. 02 |
����:
��1���ɱ��е��������ǿ�֪���ý��ָΪ________(����������������)
��2��N����ֵΪ________��
��3���˽��ָ��ͭ�����������Ƕ��٣�_________��д��������̣�
��4��������ͼ�л�������ö15g���ָ�м�ϡ����������������������仯��ϵ��ʾ��ͼ��______