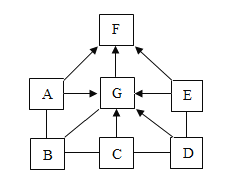
��Ŀ����
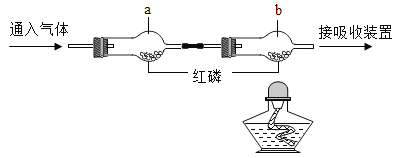
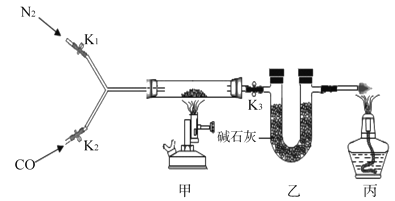
����Ŀ��ijУʵ������һƿ���õ���м����ɷ���������������ˮ��Ϊ�ⶨ���и��ɷֵ�����������ij��ȤС�鰴ͼ��ʾװ�ý���ʵ�飨װ�����������ã��̶�װ������ȥ������������м�еijɷַ�Ӧ����ʯ���������ƺ��������ƵĻ�����������ˮ�����Ͷ�����̼����
������ʵ�飩������ı�Գ���������Ӱ����Բ��ƣ�
�ٳ��������ܵ�����Ϊm1������Ʒ���벣�����У����������ܺ���Ʒ��������Ϊm2��
�����Ӻ�װ�á�����ͨ��N2����ȼ�״��ľƾ���ƣ����������й�����أ���¼�����ܺ�ʣ������������Ϊm3��
���ٴ����Ӻ�װ�ã�����ʵ�顣ͨ��CO����ȼ�����ľƾ��ƺͼ״��ľƾ���ơ����������й�����أ�Ϩ��ƾ���ƣ�����ͨ��COֱ����������ȴ���ٴμ�¼�����ܺ�ʣ������������Ϊm4��
���������ۣ�
��1�������ƾ��Ƶ�������_____��
��2����������һ����̼����������Ӧ�Ļ�ѧ����ʽΪ_____��
��3����Ʒ������������������Ϊ_____���ú���m1��m2��m3��m4�Ĵ���ʽ��ʾ����
��4�����������������û����ȫ��Ӧ����Ʒ��ˮ�����������ⶨ�����_____������ƫ������ƫС����������������
���𰸡���ȼһ����̼������β����Ⱦ 3CO+Fe2O3 2Fe+3CO2
2Fe+3CO2 ![]() ����
����
��������
��1������ʵ�������ͨ����һ����̼���ʱ����ƾ��Ƶ������ǵ�ȼһ����̼������β����Ⱦ��
��2��һ����̼����������Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ3CO+Fe2O3 2Fe+3CO2��
2Fe+3CO2��
��3������Ʒ������������������Ϊx��

![]() �����x=
�����x=![]() ��
��
��4�����������������Ӧ��ˮ�أ������������û����ȫ��Ӧ����Ʒ��ˮ�����������ⶨ��������䡣

����Ŀ�������ʯ��ʯ��Դ�ḻ��ij����С��ͬѧΪ�˲ⶨʯ��ʯ��̼��Ƶ�����������ȡij��ʯ��ʯ��Ʒ9.0g���ձ��У���50gϡ�����5�μ����ձ��У���ַ�Ӧ�����ʲ���ϡ���ᷴӦ�������ʣ������������¼���¡�
���� | 1 | 2 | 3 | 4 | 5 |
����ϡ��������/g | 10 | 10 | 10 | 10 | 10 |
ʣ���������/g | 7.0 | 5.0 | 3.0 | 1.5 | x |
����㣺
��1��x��ֵΪ_____��
��2����ȫ��Ӧ������������̼������Ϊ���٣���д��������̣�______